The Boltzmann equation gives the probability of finding a system in the state n (with energy E
Question:
The Boltzmann equation
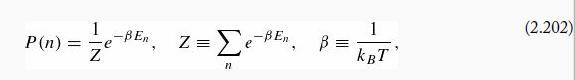
gives the probability of finding a system in the state n (with energy En), at temperature T(kB is Boltzmann's constant). The probability here refers to the random thermal distribution, and has nothing to do with quantum indeterminacy. Quantum mechanics will only enter this problem through quantization of the energies En.
(a) Show that the thermal average of the system’s energy can be written as

(b) For a quantum simple harmonic oscillator the index n is the familiar quantum number, and En = (n+1/2) ћω. Show that in this case the partition function Z is

You will need to sum a geometric series. Incidentally, for a classical simple harmonic oscillator it can be shown that Zclassical = 2π / (ωβ).
(c) Use your results from parts (a) and (b) to show that for the quantum oscillator

For a classical oscillator the same reasoning would give
![]()
(d) Acrystal consisting of N atoms can be thought of as a collection of 3N oscillators (each atom is attached by springs to its 6 nearest neighbors, along the x, y, and z directions, but those springs are shared by the atoms at the two ends). The heat capacity of the crystal (per atom) will therefore be

Show that (in this model)
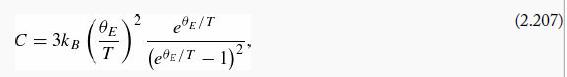
where ![]() is the so-called Einstein temperature. The same reasoning using the classical expression for E̅ yields Cclassical = 3kB, independent of temperature.
is the so-called Einstein temperature. The same reasoning using the classical expression for E̅ yields Cclassical = 3kB, independent of temperature.
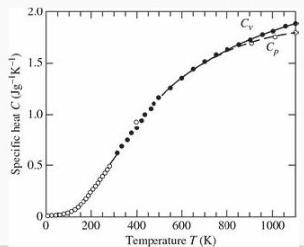
(e) Sketch the graph of C/kB versus T/θE. Your result should look something like the data for diamond in Figure 2.24, and nothing like the classical prediction.
Figure 2.24
Step by Step Answer:

Introduction To Quantum Mechanics
ISBN: 9781107189638
3rd Edition
Authors: David J. Griffiths, Darrell F. Schroeter





