At 1 bar, silver melts at 1233.95 K. The density of the liquid and solid are: You
Question:
At 1 bar, silver melts at 1233.95 K. The density of the liquid and solid are: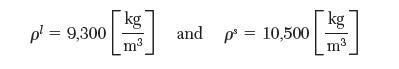
You may assume these values are constant in this problem. The entropy at the normal melting point is: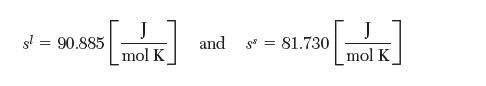
The molecular weight of silver is 107.9 [g/mol].
(a) Calculate the Gibbs energy of fusion for silver at 5,000 bar and 1400 K, assuming the entropy of each phase is constant.
(b) Which phase is stable at 5,000 bar and 1400 K? Explain.
(c) Account for the temperature dependence of entropy in the calculation for part A. The following heat capacity data are available: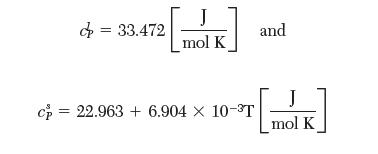
with T in [K].
(d) What is the melting temperature of silver at 1 bar? At 3,000 bar?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





