At very high temperatures, a gas can be ionized and remain in thermodynamic equilibrium. Consider the case
Question:
At very high temperatures, a gas can be ionized and remain in thermodynamic equilibrium.
Consider the case of gas containing only ions, A1. Your supervisor requests that you come up with a simple (one-parameter) equation of state for this gas. Your assistant leaves you a memo that she has fi t the PvT data to an equation of state of the form: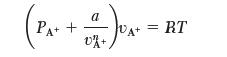
She tells you the data fi t this equation well but, unfortunately, leaves you no numbers. Your meeting with your supervisor is in 10 minutes, and your assistant is nowhere to be found! In order to be ready for the meeting, you need to answer the following questions:
(a) Is the form of this equation reasonable? Explain.
(b) What sign would you expect for the constant, a? Will this be a small or large number? Explain.
(c) What number will you use for n (it can be a fraction)? What are the units of a? Show your work.
Step by Step Answer:






