Consider a binary mixture of species a and species b at 300 K and 1 bar. The
Question:
Consider a binary mixture of species a and species b at 300 K and 1 bar. The vapor pressure of pure a at 300 K is 80 kPa. A plot of the activity coeffi cient of species a vs. mole fraction of species a is shown below. Based on this plot, answer the following questions:
(a) Specify the reference state for species a (Lewis-Randall or Henry’s). Explain.
(b) What is the value of fa?
(c) What is the value of Ha?
(d) As best as you can, come up with the Margules parameter A in the two-suffi x Margules equation![]()
(e) Consider a liquid mixture of 2 moles of a and 3 moles of b at 300 K and 1 bar. At equilibrium, what is the mole fraction of a in the vapor phase, ya?
(f) For the mixture above, determine γb based on a Lewis/Randall reference state.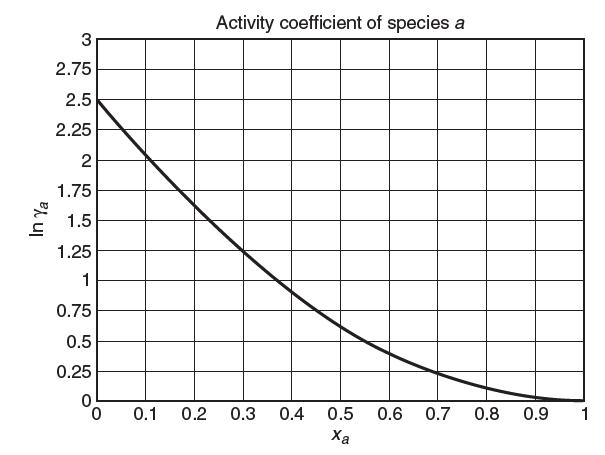
Step by Step Answer:





