Consider a cylinder fi tted with a piston that contains 2 mol of H2O in a container
Question:
Consider a cylinder fi tted with a piston that contains 2 mol of H2O in a container at 1000 K.
Calculate how much work is required to isothermally and reversibly compress this gas from 10 L to 1 L, in each of the following cases:
(a) Use the ideal gas model for water.
(b) Use the Redlich–Kwong equation to relate P, v, and T: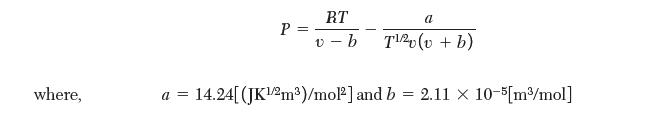
(c) Use the Steam tables.
Compare these three methods.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





