Consider the pistoncylinder assembly shown at the top of page. It is well insulated and initially contains
Question:
Consider the piston–cylinder assembly shown at the top of page. It is well insulated and initially contains two 5000-kg blocks at rest on the 0.05-m2 piston. The initial temperature is 500 K.
The ambient pressure is 5 bar. One mol of an ideal gas is contained in the cylinder. This gas is compressed in a process in which another 5000-kg block is added. The heat capacity of the gas at constant volume can be taken to have a constant value of (5/2) R, where R is the gas constant.
(a) What are the initial and fi nal pressures of the gas in the system?
(b) Do you expect the temperature to rise or fall? Explain.
(c) What is the fi nal temperature?
(d) Calculate Δssys and Δssurr.
(e) Does this process violate the second law of thermodynamics? Explain.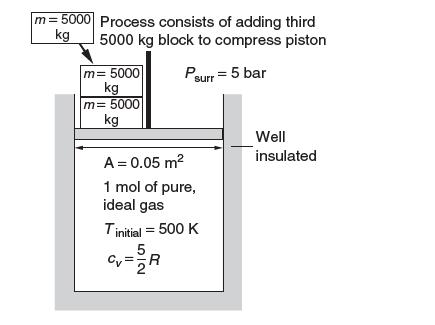
Step by Step Answer:






