Consider the piston-cylinder assembly shown below. It is well insulated and initially contains two 10,000-kg blocks at
Question:
Consider the piston-cylinder assembly shown below. It is well insulated and initially contains two 10,000-kg blocks at rest on the 0.05-m2 piston. The initial temperature is 500 K. The ambient pressure is 10 bar. Two moles of gas A are contained in the cylinder. This gas is compressed in a process where another 10,000-kg block is added. The following data are available for gas A:
(i) Ideal gas heat capacity of gas A at constant pressure:![]()
where cp is in [J/(mol K)] and T is in [K].
(ii) Gas A is can be described by the following equation of state: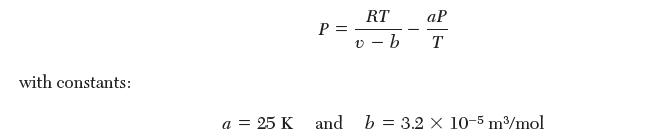
Determine the temperature of gas A after this process. Note: This compression process is not isentropic. What is the entropy change of the universe for this process?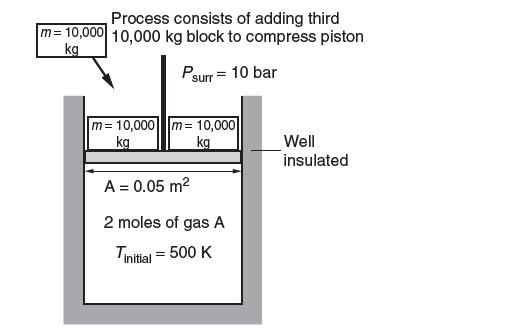
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





