Consider the piston-cylinder assembly shown below; 250 moles of gas expand isothermally after the removal of a
Question:
Consider the piston-cylinder assembly shown below; 250 moles of gas expand isothermally after the removal of a 10,000-kg block.
(a) What is the internal energy change for the expansion process?
(b) What is the entropy change of the universe for this process?
Assume that the PvT behavior can be described by the van der Waals equation with a = 0.5 3Jm3/mol2 4; b = 4 * 1025 3m3/mol4; and that the ideal gas heat capacity has a constant value of cP ideal gas 5 35J/ (mol K).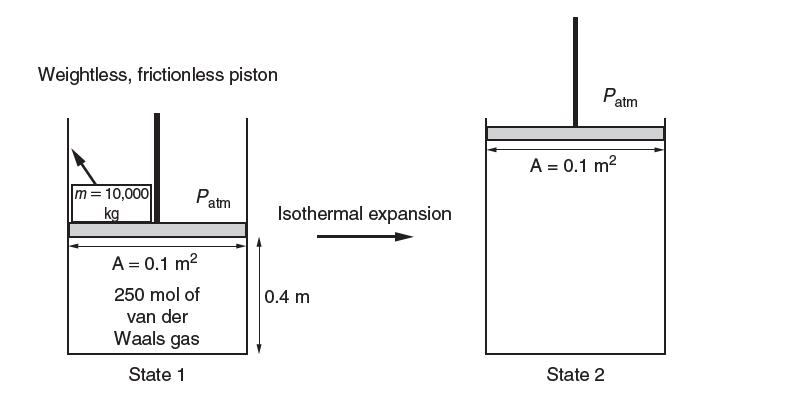
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





