Consider the reaction of CrCl2 with H2 to form solid Cr as follows: At Answer the following
Question:
Consider the reaction of CrCl2 with H2 to form solid Cr as follows: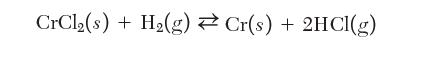
At ![]() Answer the following questions:
Answer the following questions:
(a) From these data, estimate the enthalpy of reaction.
(b) In an attempt to increase the extent of reaction, the reaction temperature is raised to 1000°C and 1 bar. At equilibrium, how much Cr is produced for every mole of H2 in the feed?
(c) Additionally, you wish to increase the extent of reaction by changing the pressure. Would you increase or decrease the pressure? Explain.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





