Develop expressions for the partial molar enthalpies of sulfuric acid and water in a binary mixture at
Question:
Develop expressions for the partial molar enthalpies of sulfuric acid and water in a binary mixture at 21°C. The pure species enthalpies are 1.596 [kJ/mol] and 1.591 [kJ/mol], respectively, and the enthalpy of mixing is given by Equation (6.24). Calculate their values for an equimolar mixture of sulfuric acid and water. ![]()
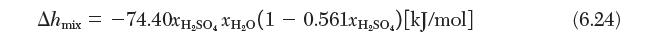
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





