Ethanol (1) and n-hexane (2) are in equilibrium at 75C. There are two liquid phases and one
Question:
Ethanol (1) and n-hexane (2) are in equilibrium at 75°C. There are two liquid phases and one vapor phase present (VLLE). The compositions of the liquid phases are: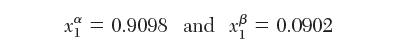
The saturation pressures at 75°C are:![]()
(a) Is the two-suffi x Margules expression ![]() a reasonable model for this system?Explain.
a reasonable model for this system?Explain.
(b) Calculate the constant, A.
(c) What is the total pressure of the system? State and justify any assumptions that you make.
(d) What is the composition of the vapor phase?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





