Fuel cells produce electricity from hydrogen. The life of the fuel cell depends on producing relatively pure
Question:
Fuel cells produce electricity from hydrogen. The life of the fuel cell depends on producing relatively pure hydrogen. Methane (natural gas) is often used as a feed to produce hydrogen.
Consider the production of hydrogen (H2) by the dissociation of methane (CH4) into solid carbon (C). The process can be described by the following chemical reaction:![]()
The temperature is 1000 K and the pressure is 1000 Pa. You may assume that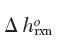
does not change with temperature.
(a) What is the equilibrium constant at 298 K?
(b) What is the equilibrium constant at 1000 K?
(c) What is the maximum amount of H2 that can be produced per mole of CH4 in the feed?
(d) Why is this reaction run at 1000 K instead of 298 K?
(e) Why is this reaction run at 1000 Pa instead of 1 bar?
Step by Step Answer:






