mixture of toluene (1) and polystyrene (2) is placed in an evacuated closed container (there is no
Question:
mixture of toluene (1) and polystyrene (2) is placed in an evacuated closed container (there is no air in the container). The container is then brought to 20.57 bar and 301°C where the liquid solution is in equilibrium with pure toluene vapor. Because polystyrene is large, you can assume no polystyrene is in the vapor phase. Calculate the mole fraction of toluene in the liquid.
You may neglect the Poynting correction. The following plot shows the activity coeffi cient of toluene in polystyrene.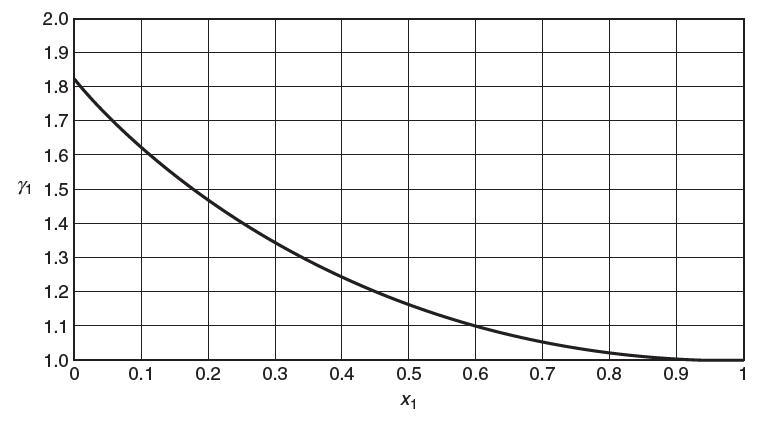
Step by Step Answer:
Related Book For 

Question Posted:




