Solve the multiple chemical reaction equilibrium problem in Example 9.19 at 800 K using the following set
Question:
Solve the multiple chemical reaction equilibrium problem in Example 9.19 at 800 K using the following set of independent reactions: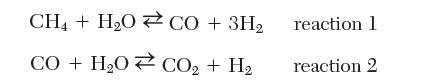
Example 9.19
Consider a system initially charged with 1 mole of pure I2 that is maintained at 1300 K and 1 bar in which the following dissociation reaction occurs: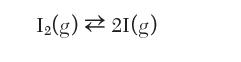
For monatomic iodine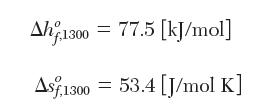
Plot ΔH, TΔS and ΔG as a function of extent of reaction. What is the equilibrium conversion?
Step by Step Answer:
Related Book For 

Question Posted:




