The fi eld of nanotechnology is an emerging area for chemical and biological engineers. Consider nickel nanoparticles
Question:
The fi eld of nanotechnology is an emerging area for chemical and biological engineers. Consider nickel nanoparticles for use as a catalyst to grow carbon nanotubes. The normal melting temperature, Tm, of nickel is 1728 K. In a nanoparticle, the curved surface leads to a force acting tangentially to the surface of the particles, which changes the Gibbs energy of the solid. The magnitude of this effect is determined by the surface tension, s. The differential change in Gibbs energy can be written to account for surface tension in terms of curvature, or inverse radius (1/R). In this case, the fundamental property relation given by Equation (5.9)
can be modifi ed as follows: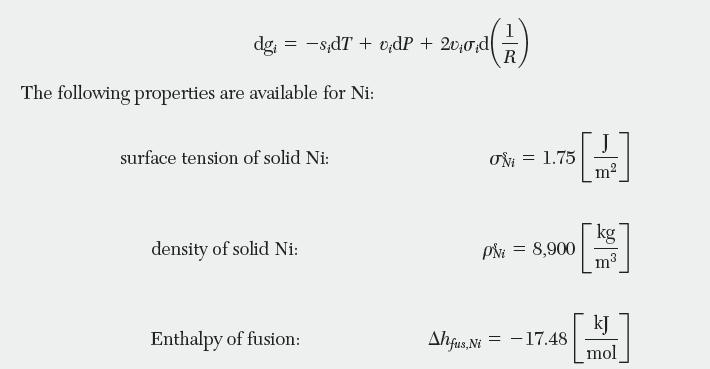
Consider Ni nanoparticles with radius of approximately 2 nm (2 * 1029 m). You are interested in whether they might melt when they are processed at 750°C and 1 atm. For simplicity, you may assume that the system contians only pure nickel nanoparticles. At 750°C and 1 atm, what is the phase of pure 2 nm Ni nanoparticles at equilibrium? State any assumptions that you make.
Step by Step Answer:






