The following diagram shows the normal melting point of pure solid 1 to be Tm. Consider now
Question:
The following diagram shows the normal melting point of pure solid 1 to be Tm. Consider now that the same pure solid 1 is in a liquid mixture with four species 1, 2, 3, and 4 as shown on the right. How does the temperature at which 1 will be in equilibrium with liquid, T, compare to the case on the left (T Tm, or you cannot tell without more information)? Explain your answer.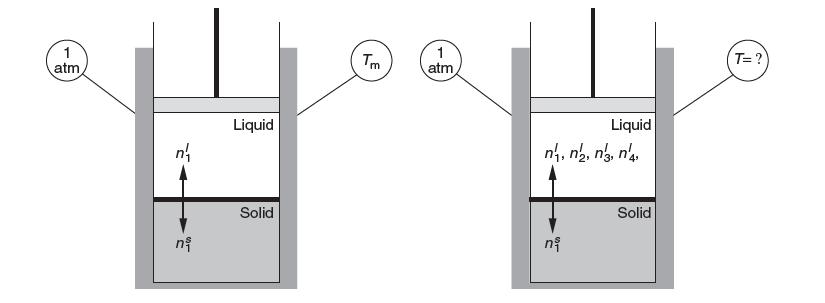
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





