We are interested in the thermodynamic properties of a strip of rubber as it is stretched (see
Question:
We are interested in the thermodynamic properties of a strip of rubber as it is stretched (see below). Consider n moles of pure ethylene propylene rubber (EPR) that has an unstretched length z0.
If it is stretched by applying a force F, it will obtain an equilibrium length z, given by: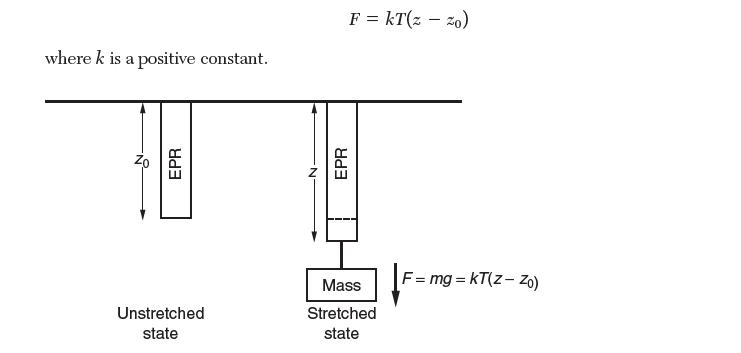
The heat capacity of unstretched EPR is given by: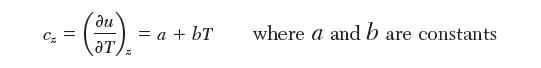
(a) Come up with fundamental property relations for dU and dA for this system, where A is the Helmholtz energy (A = U - TS). Recall from mechanics that the work required for a reversible elastic extension is given by: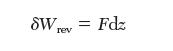
(b) Develop an expression that relates the change in entropy to the changes in temperature and length, that is, the independent properties z and T (and constants
a, b, k, n and z0). In other words, for S = S(T, z) , fi nd dS.
(c) Develop an expression that relates the change in internal energy to the changes in temperature and length. For U = U(T, z) , fi nd dU.
(d) Consider the relative energetic and entropic contributions to the isothermal extension of EPR.
The energetic force (the component of the force that, on isothermal extension of the rubber, increases the internal energy) is given by: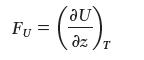
while the entropic force is given by: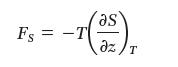
Come up with expressions for FU and Fs for EPR.
(e) If the change from the unstretched state to the stretched state above occurred adiabatically, would the temperature of the EPR go up, stay the same, or go down? Explain.
Step by Step Answer:






