A sealed 1-L flask is filled with 1 mole of H 2 (g) and 1 mole of
Question:
A sealed 1-L flask is filled with 1 mole of H2(g) and 1 mole of I2(g). The reversible reaction H2(g) + I2(g) → 2HI(g) ensues. A plot showing what happens to the HI concentration as a percent of the gas mixture over time is shown below.
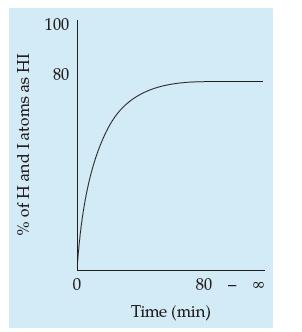
Now imagine repeating the experiment in the same sealed flask, but this time filling the flask with 2 moles of HI(g) and no H2(g) or I2(g). Sketch the %HI plot versus time that one might expect, and then justify it with an explanation.
Step by Step Answer:
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:




