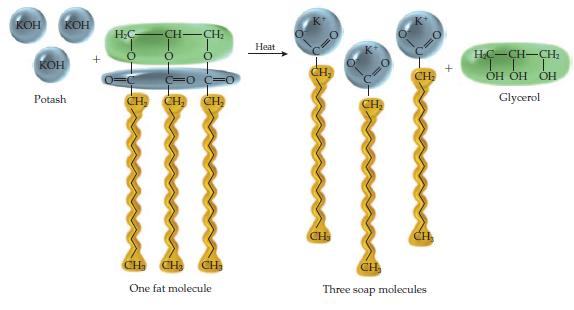
Consider the soap molecules at the top of page 495 and the ionic compound potassium acetate. (a)
Question:
Consider the soap molecules at the top of page 495 and the ionic compound potassium acetate.
(a) Draw a dot diagram for the acetate ion, and then place a K+ ion next to the O bonded to C by a single bond.
(b) What is the similarity between potassium acetate and a soap molecule? How do they differ?
(c) Hard water often contains calcium ions, which react with soap molecules to form a solid soap scum. Draw a soap scum molecule, using a soap molecule containing ten C atoms. What is the molecular formula of this soap scum molecule?
Data from Page 495
Step by Step Answer:
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:




