Some alcohols are quite soluble in water. For example, isopropyl alcohol, shown below, is sold as an
Question:
Some alcohols are quite soluble in water. For example, isopropyl alcohol, shown below, is sold as an aqueous solution we call rubbing alcohol. Show how a water molecule would be attracted to isopropyl alcohol, and name the strongest intermolecular force involved. Would you call this interaction hydration?
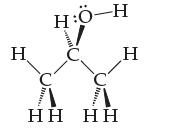
Transcribed Image Text:
Н HQ-H н C C НН НН Н
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)

Answered By

Shameen Tahir
The following are details of my Areas of Effectiveness. The following are details of my Areas of Effectiveness English Language Proficiency, Organization Behavior , consumer Behavior and Marketing, Communication, Applied Statistics, Research Methods , Cognitive & Affective Processes, Cognitive & Affective Processes, Data Analysis in Research, Human Resources Management ,Research Project,
Social Psychology, Personality Psychology, Introduction to Applied Areas of Psychology,
Behavioral Neurosdence , Historical and Contemporary Issues in Psychology, Measurement in Psychology, experimental Psychology,
Business Ethics Business Ethics An introduction to business studies Organization & Management Legal Environment of Business Information Systems in Organizations Operations Management Global Business Policies Industrial Organization Business Strategy Information Management and Technology Company Structure and Organizational Management Accounting & Auditing Financial Accounting Managerial Accounting Accounting for strategy implementation Financial accounting Introduction to bookkeeping and accounting Marketing Marketing Management Professional Development Strategies Business Communications Business planning Commerce & Technology Human resource management General Management Conflict management Leadership Organizational Leadership Supply Chain Management Law Corporate Strategy Creative Writing Analytical Reading & Writing Other Expertise Risk Management Entrepreneurship Management science Organizational behavior Project management Financial Analysis, Research & Companies Valuation And any kind of Excel Queries.
4.70+
16+ Reviews
34+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
In the diagram below, each circle represents a glycosyl unit. By the action of branching enzyme, show the most branched structure this molecule can assume. Indicate the reducing and nonreducing ends...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
(a) Name the strongest intermolecular force in CH 3 OH, CH 3 Cl, CH 3 CH 3 , and CH 3 CH 2 CH 3 . (b) Rank these molecules from lowest to highest boiling point.
-
Write a report on Home and Automobile: These are two of the most important financial purchases we will make. These decisions, especially housing, will affect much of your ability to meet your...
-
Why is the accounting for a manufacturing business more complicated than that for a merchandising business?
-
List the characteristics of simulation.
-
Refer to the information in Exercise 17-7 to answer the following requirements. Required 1. Using ABC, compute the overhead cost per unit for each product line. 2. Determine the total cost per unit...
-
Information related to Kerber Co. is presented below. 1. On April 5, purchased merchandise from Wilkes Company for $23,000, terms 2/10, net/30, FOB shipping point. 2. On April 6, paid freight costs...
-
1. A ray of light enters glass (index 1.570) from air at an incident angle of 25. Find the angles of refraction and of deviation. 2. A light ray is directed through air (index 1.000) at a 25 angle of...
-
What is the name of the attractive force between dissolved Na + ions and water molecules? Diagram this force, showing how a water molecule would approach an Na + ion. Do the same for a Cl ion.
-
When NaCl dissolves, what helps keep the dissolved Na + and Cl ions from coming back together and reforming the lattice, precipitating the solid?
-
A company acquires a machine with an estimated 10-year service life. If the company uses the Accelerated Cost Recovery System depreciation method instead of the straightline method: a. Income will be...
-
In the table below are the calculations of the total and average cost of an engineering equipment during eight years of service. Explain by drawing the relationship between the average annual cost...
-
Define the concept of a buffer and elucidate its significance in the preservation of biological macromolecules, particularly enzymes, within a complex biochemical milieu.
-
How have technological innovations aided the wealthy to avoid paying taxes?
-
It has been argued that "regional integration allows countries to overcome costly divisions through integrating goods, services and factors' markets, thus facilitating the flow of trade, capital,...
-
The statement that best describes the end behaviour of the graph of g(x) = x(x-3)(x+2)(x-1) is the graph extends Select one: a. upward into quadrant I and downward into quadrant III O b. downward...
-
The following list shows the top six pharmaceutical companies in the United States and their sales figures ($ millions) for a recent year. Use this information to construct a pie chart and a bar...
-
CRUZ, INC. Comparative Balance Sheets December 31, 2015 CRUZ, INC. Income Statement For Year Ended December 31, 2015 Required Use the indirect method to prepare the cash provided or used from...
-
The New Jersey barrier is commonly used during highway construction. Determine its weight per foot of length if it is made from plain stone concrete. 4 in.- 75 55 12 in. 6 in. 24 in.
-
The floor of a light storage warehouse is made of 150-mm-thick lightweight plain concrete. If the floor is a slab having a length of 7 m and width of 3 m, determine the resultant force caused by the...
-
The prestressed concrete girder is made from plain stone concrete and four -in. cold-form steel reinforcing rods. Determine the dead weight of the girder per foot of its length. 8 in. 6 in. 20 in. 6...
-
K a. Why does "margin of error" have no relevance for confidence interval estimates of o or o? b. What is wrong with expressing the confidence interval of 6.9 bpm
-
point Which is NOT one of the 3 Cs of user story creation? Conversation-The user story is supported by a conversation or is a starting point for a conversation between business and project teams....
-
Solve the equation x-2 6x-5 = 219 x-2 X = Submit Question

Study smarter with the SolutionInn App


