Using the EMF series on page 391, decide which of the following redox reactions is spontaneous. Explain
Question:
Using the EMF series on page 391, decide which of the following redox reactions is spontaneous. Explain your answer.
(a) 3K + Al3 + → Al + 3K+
(b) Al + 3K+ → 3 K + Al3 +
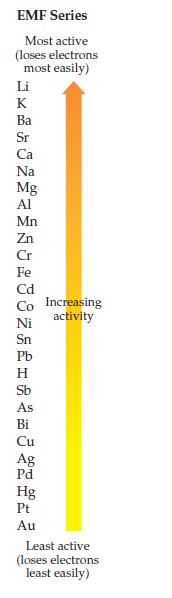
Transcribed Image Text:
EMF Series Most active (loses electrons most easily) Li HKkxGMSIMAGRGOMADHA &斑Q8HHH加 Ba Sr Ca Na Mg Al Mn Zn Cr Fe Cd Co Increasing Ni Sn Pb Н As Bi Cu Ag Pd Hg Pt Au activity Least active (loses electrons least easily)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)

Answered By

Shaira grace
I have experience of more than ten years in handing academic tasks and assisting students to handle academic challenges. My level of education and expertise allows me communicate eloquently with clients and therefore understanding their nature and solving it successfully.
5.00+
4+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
Review the information below: Cash balance per bank statement $9,525 Cash balance per books $10,094 NSF check $245 Service charge $35 Outstanding checks $1,382 Collection of notes receivable $884...
-
Using the EMF series on page 391, decide which of the following redox reactions is spontaneous. Explain your answer. (a) 3Ag + Au 3+ Au + 3 Ag + (b) Au + 3Ag + 3Ag + Au 3+ EMF Series Most active...
-
Suppose gold were not available for a wedding ring, but you still wanted a ring that would last forever and not corrode. According to the EMF series on page 391, what would be a good alternative...
-
For the planning process, it is helpful to have employee census information for Question 3 options: a) at least the last five years and projections for the future. b) all current employees younger...
-
Sunshine Baking Company is a diversified food products company with three operating divisions organized as investment centers. Condensed data taken from the records of the three divisions for the...
-
(a) Sketch the electric eld pattern set up by a positively charged hollow sphere. Include regions inside and regions outside the sphere. (b) A conducting cube is given a positive charge. Sketch the...
-
A \(1500-\mathrm{kg}\) truck and a \(1000-\mathrm{kg}\) car are parked with their rear bumpers nearly touching each other in a level parking lot. Both vehicles have their brakes off so that they are...
-
A 10-year U.S. Treasury bond with a face value of $10,000 pays a coupon of 5.5% (2.75% of face value every six months). The semiannually compounded interest rate is 5.2% (a six-month discount rate of...
-
Verizon Communications Inc. had $13.44 billion in FCFF in the most recent year. If the firm's expected growth rate is 5%, its WACC is 10% and the cost of equity is 13%, what is the value of Verizon?
-
What happens when you place a less active metal in a solution of ions of a more active metal?
-
What happens when you place an active metal in a solution of ions of a less active metal?
-
Home Depot Inc. misjudged the market in China. The worlds largest home improvement chain entered the market in China in 2006 and decided to leave six years later. It was not able to sell its...
-
Concerning multiuser systems 1 a) What are multi user systems? b) Why are they successful? 2) Concerning Linux a) What programming language is Linux written in? b) How did this language influence the...
-
Do you think that the positivity of the office space is mostly on the employer or employee
-
You're assigned a group project and asked to come up with the following bold items for the team. Desirable team behaviors and consequences for non compliance, team conflict management plan, and team...
-
Consider the 3 generators with the characteristics given in Tab. 1, they must satisfy the load requirement in Tab. 2. Unit Pmin Pmax Marginal cost ($/MWh) Min up time (h) ($) No-load cost ($) 1 30 3...
-
Managing Linux users - Some of the topics in this week's material were around managing Linux user accounts. What are some of the concerns around this and some techniques to support managing Linux...
-
What is the difference between an expense and a loss?
-
Without solving, determine the character of the solutions of each equation in the complex number system. 3x 2 3x + 4 = 0
-
From the image configuration determine the shape of the mirror hanging on the back wall in van Eycks painting of John Arnolfini and His Wife (Fig. P.5.75). Fig. P.5.75
-
The image of a red rose is formed by a concave spherical mirror on a screen 100 cm away. If the rose is 25 cm from the mirror, determine its radius of curvature.
-
A thin lens having a focal length of +50.0 cm is positioned 250 cm in front of (i.e., to the left of) a plane mirror. An ant sits on the central axis 250 cm in front of (i.e., to the left of) the...
-
Given table below answer the questions. Beginning inventory Merchandise Finished goods Cost of merchandise purchased Cost of goods manufactured Ending inventory Merchandise Finished goods Unimart...
-
You are provided with the following data. Sales Variable costs: $ 16,528 Direct Materials Direct Labor Manufacturing Overhead Variable selling Variable administrative sssss $ 1,764 $ 2,587 $ 952 $...
-
Saved Help Save & Exit Submi A manufacturer reports the following information. The company applies overhead using a predetermined overhead rate of 55% of direct labor cost. Inventories Raw materials...

Study smarter with the SolutionInn App


