We left three possible situations out of Table 6.2: 1. Four electron groups (SN = 4): one
Question:
We left three possible situations out of Table 6.2:
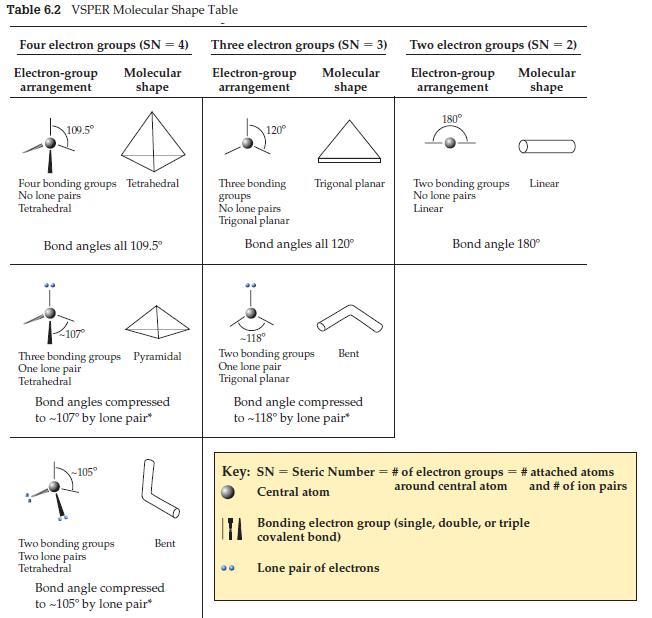
1. Four electron groups (SN = 4): one bonding group and three lone pairs.
2. Three electron groups (SN = 3): one bonding group and two lone pairs.
3. Two electron groups (SN = 2): one bonding group and one lone pair.
These are considered trivial cases. Why? What shape(s) do these bonding situations give rise to?
Transcribed Image Text:
Table 6.2 VSPER Molecular Shape Table Four electron groups (SN = 4) Molecular shape Electron-group arrangement 109.5⁰ Four bonding groups Tetrahedral No lone pairs Tetrahedral Bond angles all 109.5⁰ Three bonding groups Pyramidal One lone pair Tetrahedral Bond angles compressed to ~107° by lone pair* -105° Two bonding groups Two lone pairs Tetrahedral Bent Bond angle compressed to ~105° by lone pair* Three electron groups (SN = 3) Electron-group Molecular arrangement shape 120° Three bonding groups No lone pairs Trigonal planar Trigonal planar Bond angles all 120° -118° Two bonding groups One lone pair Trigonal planar Bent Bond angle compressed to ~118° by lone pair* Two electron groups (SN = 2) Electron-group Molecular shape arrangement 180° Two bonding groups No lone pairs Linear Linear Bond angle 180° Key: SN = Steric Number = # of electron groups = # attached atoms and # of ion pairs around central atom Central atom Bonding electron group (single, double, or triple covalent bond) Lone pair of electrons
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 25% (4 reviews)
These are trivial because they all represent a central at...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
A student forgets that the N in ammonia, NH 3 , has a lone pair as well as its three single bonds. After checking Table 6.2, he mistakenly draws the moleculethree bonding groups, no lone pairs as...
-
Defining the Problem (1). Lead is an environmental pollutant especially worthy of attention because of its damaging effects on the neurological and intellectual development of children. Morton et al....
-
The Crazy Eddie fraud may appear smaller and gentler than the massive billion-dollar frauds exposed in recent times, such as Bernie Madoffs Ponzi scheme, frauds in the subprime mortgage market, the...
-
A decision maker is working on a problem that requires her to study the uncertainty surrounding the payoff of an investment. There are three possible levels of payoff $1,000, $5,000, and $10,000. As...
-
What three manufacturing budgets can be prepared subsequent to preparation of the production budget?
-
Stenback Company is one of the worlds leading corn refiners. It produces two joint productscorn syrup and corn starchusing a common production process. In July 2014, Stenback reported the following...
-
Hooters Restaurant in Myrtle Beach, South Carolina, used an alternative dispute resolution program, a program to resolve disputes outside the traditional court system. Employees of Hooters had to...
-
Peoria Corp. just completed another successful year, as indicated by the following income statement: Presented here are comparative balance sheets: Other information is as follows: a. Dividends of...
-
The graph below shows the number of people who consume different numbers of lollipops per year. What is the median number of lollipops consumed? Number of People 3 27 24 21 18 15 12 96 Number of...
-
Describe the geometry of the electron groups and name the molecular shape resulting from that geometry. Also, draw the molecule, and label the size of all bond angles in your drawing. C 2 Cl 2 ,...
-
The steric number of an atom (a) Is equal to the number of electrons on that atom. (b) Is equal to the number of electron pairs on that atom. (c) Is equal to the number of lone pairs on the atom. (d)...
-
Starr Mfg.'s predetermined overhead rate is 200% of direct labor. Information on the company's production activities during September 2015 follows. a. Purchased raw materials on credit, $125,000. b....
-
Define the EBIT-EPS indifference point.
-
Within the financial markets, explain what we mean by private placements and name the advantages and disadvantages.
-
In early 2016, Alfa Corporation issued new common stock at a market price of $30. Dividends last year were $2.00 and are expected to grow at an annual rate of 3 percent forever. Floatation costs will...
-
What is a scenario analysis? What is a sensitivity analysis? When would you perform a sensitivity analysis?
-
Name the benefits derived from the existence of stock exchanges.
-
List the four types of bonus payment options and explain how they differ. How does each achieve or not achieve the three objectives of management compensation?
-
Floyd Distributors, Inc., provides a variety of auto parts to small local garages. Floyd purchases parts from manufacturers according to the EOQ model and then ships the parts from a regional...
-
Explain why the speed of the particle needs to be taken into account in calculating the probability for transmission over a step potential.
-
The reflection probability from a step potential was calculated for E > V 0 in Section 16.5. Is Equation (16.18) valid for E < V 0 ? What information can you extract from Figure 16.1 that will allow...
-
Figure 16.17 shows that atomic level resolution is only attainable in the repulsive portion of the tipsurface potential. What does this tell you about the range of the attractive and repulsive parts...
-
1. Can a person freely dispose of his/her property through a will and what are the key requirements for a will to be considered valid? 2. What role does the Family Code play in the succession of...
-
What is the output of the code snippet given below? int i; int j = 0; for (i=0; i < 6; i++) { if (i % 2 (0) { - i = i + 2; } j++; else { i++; j = j + 2; j++; } }
-
How many times does the following loop execute? double num; double sum = 0; for (int i = 0; i <10; i++) { cout < < "Please enter a number: "; cin >> num; if (sum num) { sum = num; } if (i == 5) { i =...

Study smarter with the SolutionInn App


