Based on the general trends in the periodic table, predict which element in each of the following
Question:
Based on the general trends in the periodic table, predict which element in each of the following pairs has the higher ionization energy:
(a) Li or Na
(b) O or F.
Periodic Table:
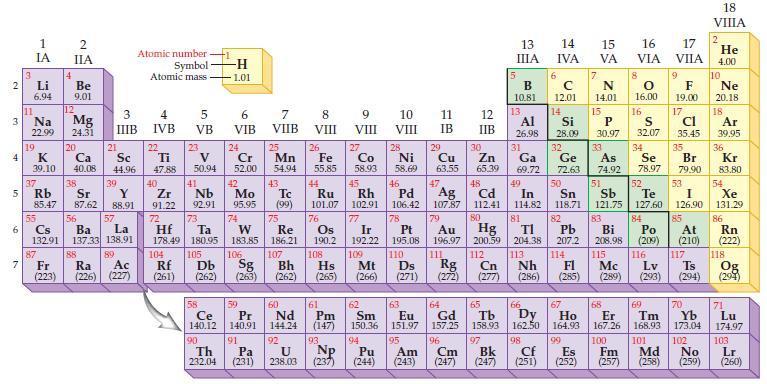
Transcribed Image Text:
2 3 4 in 6 3 7 Li 6.94 11 1 IA Na 22.99 19 G 85.47 55 4 37 38 5 Rb Sr 87.62 2 IIA Be 9.01 K Ca 39.10 40.08 87 12 Mg 24.31 20 21 88 Sc 44.96 56 Cs Ba 132.91 137.33 138.91 39 3 IIIB Y 88.91 57 Lo La 89 O Atomic number Symbol Atomic mass Fr Ra Ac (223) (226) (227) 4 IVB 22 Ti 47.88 40 Zr 91.22 72 5 VB 23 V 50.94 41 Nb 92.91 73 Hf Ta 178.49 180.95 104 105 Rf (261) 58 -H 1.01 Ce 140.12 90 Th 232.04 6 VIB 24 Cr 52.00 42 Mo 95.95 74 W 183.85 7 VIIB 91 25 Pa (231) Mn 54.94 43 Tc (99) 75 Re 186.21 59 60 Pr Nd 140,91 144.24 107 8 VIII 92 U 238.03 26 Fe 55.85 44 Ru 101.07 76 Os 190.2 108 77 78 Ir 192.22 109 Mt Pt 195.08 110 Ds 106 Bh Hs Rg ᎠᏏ Sg Cn (262) (263) (262) (265) (266) (271) (272) (277) 9 VIII 93 Co 58.93 Np (237) 10 VIII 28 Ni 58.69 45 46 Rh Pd 102.91 106.42 11 IB 29 Cu 63.55 47 12 IIB 30 111 Zn 65.39 5 13 ΠΙΑ B 10.81 48 Ag 107.87 79 Cd 112.41 80 Hg Au Pb 196.97 200.59 204.38 207.2 112 14 IVA 6 C 12.01 14 F 63 64 65 66 67 61 62 Pm Sm Eu Gd Tb Dy Ho (147) 150.36 151.97 157.25 158.93 162.50 164.93 96 97 Cm Bk (247) (247) 98 99 94 95 Pu Am (244) (243) Cf Es (251) 114 113 Nh FI (286) (285) 7 15 VA 13. 15 Al Si P 26.98 28.09 30.97 31 Se 49 32 33 Ga Ge As 69.72 72.63 74.92 78.97 50 51 52 In Sn Sb Te 114.82 118.71 121.75 127.60 81 82 83 84 Bi Po 208.98 (209) 115 116 Mc Lv Ts (289) (293) (294) N 14.01 16 17 VIA VIIA 8 9 0 F 16.00 19.00 17 cl 35.45 16 S 32.07 34 35 Br 79.90 53 I 126.90 85 At (210) 117 69 70 Tm Yb 168.93 173.04 101 102 Md No (252) (257) (258) (259) 68 Er 167.26 100 Fm 18 VIIIA 2 He 4.00 10 Ne 20.18 18 Ar 39.95 36 Kr 83.80 54 Xe 131.29 86 Rn (222) 118 Og (294) 71 Lu 174.97 103 Lr (260)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
Lets refer to a periodic table and apply the general trends in ionization energy which increases up ...View the full answer

Answered By

Joseph Mwaura
I have been teaching college students in various subjects for 9 years now. Besides, I have been tutoring online with several tutoring companies from 2010 to date. The 9 years of experience as a tutor has enabled me to develop multiple tutoring skills and see thousands of students excel in their education and in life after school which gives me much pleasure. I have assisted students in essay writing and in doing academic research and this has helped me be well versed with the various writing styles such as APA, MLA, Chicago/ Turabian, Harvard. I am always ready to handle work at any hour and in any way as students specify. In my tutoring journey, excellence has always been my guiding standard.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Find the total capacitance CT of the network in figure. 4 F: - 12 F 1 F 3 F 2 F
-
Based on the general trends in the periodic table, predict which element in each of the following pairs has the higher ionization energy: (a) Na or Mg (b) O or S. Periodic Table: 2 3 4 15 6 7 3 11...
-
Based on the general trends in the periodic table, predict which element in each of the following pairs has the most metallic character: (a) Sn or Pb (b) Ag or Sr (c) Al or B (d) Ga or Ge. Periodic...
-
Calculate the component of v = ( 2, 1/2, 3) along w = (1, 2, 2).
-
Multiple Choice. Choose the best answer. The organization assigned primary responsibility for establishing accounting and financial reporting standards for health care organizations is the: a....
-
Earnings per Share and Dividend Payout Ratio Compute (1) Earnings per share and (2) Dividend payout ratio for Companies S and T. Indicate which of the two companies is more likely to be an older...
-
Why is the figure for operating profit important? (a) This is the figure used for calculating federal income tax expense. (b) The figure for operating profit provides a basis for assessing the...
-
Archway Tech Corp. manufactures surveying equipment. Journalize the entries to record the following selected equity investment transactions completed by Archway during 2012: Feb. 2. Purchased for...
-
4. The two pentagons shown below are similar. m h 4 in. 12 in. 3 in. X Use the given information to find the exact values of the indicated unknown quantities x, h, 1, and m.
-
Which of the following elements has the highest ionization energy? (a) H (b) He (c) Ne (d) Cl (e) F.
-
Draw the electron dot formula for each of the following elements: (a) Si (b) Xe.
-
Describe three analytical techniques for financial statement analysis.
-
For each of the following pairs of hypotheses, explain what the null hypothesis should be. (a) Not guilty versus guilty in a court case. (b) Cage is safe versus cage is unsafe when testing the safety...
-
A survey wherein 90 employees were randomly drawn shows that the average number of sick days taken by employees each year is 5.4 days. The number of sick days follows a normal distribution with a...
-
In 2016, BMW celebrated its 100th anniversary. With changes in digital technology, lifestyles, and regulatory requirements, the car industry faces the challenge that people of the future may no...
-
As CEO of Chiquita, you are eager to promote CSR efforts, such as complying with the Social Accountability 8,000 labor rights standard and Rain Forest environmental standard. However, you are...
-
When we construct a 90 % confidence interval for, say, a mean, we build a range that has an upper bound and a lower bound, and we write the confidence interval as P(lower bound < mean < upper bound)...
-
Study the Excel output shown in Problem 13.13. Comment on the overall strength of the regression model in light of S, R2, and adjusted R2.
-
A sprinkler head malfunctions at midfield in an NFL football field. The puddle of water forms a circular pattern around the sprinkler head with a radius in yards that grows as a function of time, in...
-
Assume the same information as in P21-4. (Round all numbers to the nearest cent.) (a) Assuming the lessors accounting period ends on September 30, answer the following questions with respect to this...
-
The following facts pertain to a non-cancelable lease agreement between Faldo Leasing Company and Vance Company, a lessee. The lessee assumes responsibility for all executory costs, which are...
-
Ludwick Steel Company as lessee signed a lease agreement for equipment for 5 years, beginning December 31, 2010. Annual rental payments of $40,000 are to be made at the beginning of each lease year...
-
Three highly similar and competitive income-producing properties within two blocks of the subject property have sold this month. All three offer essentially the same amenities and services as the...
-
Suppose that an income producing property is expected to yield cash flows for the owner of $10,000 in each of the next five years, with cash flows being received at the end of each period. If the...
-
Mia wants to invest in Government of Canada bonds that have a par value of $20,000 and a coupon rate of 5.6 percent. The bonds have a 9-year maturity, and Mia requires an 8 percent return. How much...

Study smarter with the SolutionInn App


