Given the density of silver (10.5 g/cm 3 ) and gold (19.3 g/cm 3 ) in Group
Question:
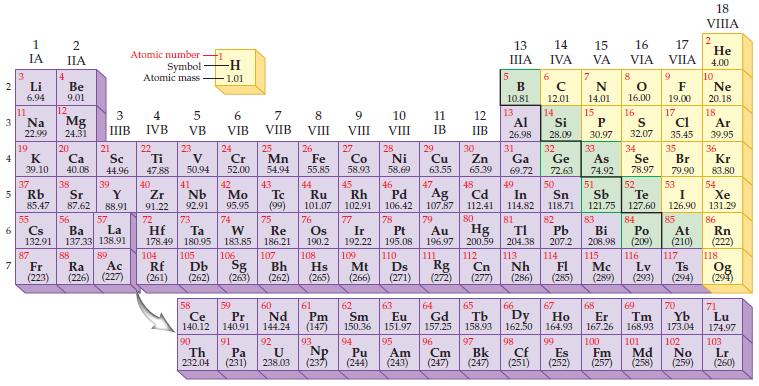
Given the density of silver (10.5 g/cm3) and gold (19.3 g/cm3) in Group IB/11, estimate the density for the synthetic element roentgenium, which is below gold in the periodic table.
Periodic Table:
Transcribed Image Text:
2 3 4 10 6 3 7 11 1 IA Li 6.94 Na 22.99 19 37 5 Rb K 39.10 4 87 IIA Fr (223) Be 9.01 12 Mg 24.31 20 38 Ca Sc 40.08 44.96 21 Sr Y 85.47 87.62 88.91 55 56 La Cs Ba 132.91 137.33 138.91 88 3 4 IIIB IVB 39 57 89 Atomic number Symbol Atomic mass Ra Ac (226) (227) 22 Ti 47.88 40 Zr 91.22 72 5 VB 104 23 V 50.94 41 73 Hf Ta 178.49 180.95 Nb 92.91 105 -H 1.01 58 Ce 140.12 90 Th 232.04 6 VIB 24 Cr 52.00 42 Mo 95.95 74 106 Rf Db Sg (261) (262) (263) 59 Pr 140.91 91 7 VIIB Pa (231) 25 W Re 183.85 186.21 Mn 54.94 43 Tc (99) 75 107 60 Nd 144.24 8 VIII 92 26 Fe 55.85 44 Os 190.2 108 Ru Rh 101.07 102.91 76 61 Pm (147) 9 VIII Bh Hs Mt (262) (265) (266) 93 27 U NP 238.03 (237) Co 58.93 45 77 Ir 192.22 109 28 Ni 58.69 13 10 VIII 11 IB 13 IIIA 12 IIB 62 Sm 150.36 94 95 Pu Am (244) (243) B 10.81 14 15 IVA 7 N 14.01 15 P Al Si 26.98 28.09 30.97 17 16 VA VIA VIIA 6 C 12.01 14 16.00 16 83 Bi 208.98 115 S 32.07 29 30 34 31 32 33 Cu Zn Ga Ge As Se 63.55 65.39 69.72 72.63 74.92 78.97 84 Po (209) 116 66 67 68 63 64 65 69 Eu Gd Tb Dy Но Er Tm 151.97 157.25 158.93 162.50 164.93 167.26 168.93 96 97 98 99 100 Bk Cf Es Fm (247) (251) (252) (257) 101 Cm (247) 46 47 51 48 49 50 52 Ag Cd In Sn Sb Te 107.87 112.41 114.82 118.71 121.75 127.60 80 79 Pd 106.42 78 Pt 195.08 110 Au Hg 196.97 200.59 111 112 Cn 81 82 TI Pb 204.38 207.2 113 114 Nh Fl Ds Rg Mc Lv Ts (271) (272) (277) (286) (285) (289) (293) (294) 9 Md (258) F 19.00 17 Cl 35.45 35 Br 79.90 53 I 126.90 85 At (210) 117 70 Yb 173.04 102 18 VIIIA 2 He 4.00 10 Ne 20.18 18 Ar 39.95 36 Kr 83.80 54 Xe 131.29 86 Rn (222) 118 Og (294) 71 Lu 174.97 103 No Lr (259) (260)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
Given the density of Ag ...View the full answer

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
KYC's stock price can go up by 15 percent every year, or down by 10 percent. Both outcomes are equally likely. The risk free rate is 5 percent, and the current stock price of KYC is 100. (a) Price a...
-
b. Calculate the value of a $4,000-par-value bond paying quarterly interest at an annual coupon interest rate of 5% and having 10 years until maturity if the required return on similar-risk bonds is...
-
Assume the state of the Book of Orders. What transactions are performed and what is the status of the book after the following orders. a) market order to buy 1700 shares. b) limit order to buy 4000...
-
A managers key task is to balance which four customer service factors against which six logistics cost factors?
-
Explain the conditions that must exist for a public or private college or university to avoid accounting recognition of the value of its collections of art, historical treasures, and similar assets.
-
Barnes knows that SKI sells on the same credit terms as other firms in its industry. Use the ratios presented earlier to explain whether SKIs customers pay more or less promptly than those of its...
-
To have the best resolution, should an electron microscope use very fast electrons or very slow electrons? Explain.
-
A published study of a chemical reaction A ? P, indicates that if the reactor initially contains A at a concentration C A0 (g/L) and the reaction temperature, T, is kept constant, then the...
-
(a) Prove that for all n = N, n(n + 1) : n(n+1)(n+2) = 3 (n 1)(n)(n + 1) 3 (b) Let n N. Using part (a), the properties of summation notation and without using induction, prove that i(i + 1) = i=1 n(n...
-
Predict the missing value (?) for each physical property listed below. The (a) Atomic radius, (b) Density, (c) Melting point are given for two of three alkaline earth metals in Group IIA/2. Element...
-
Which period represents the smallest atomic radius? Which group represents the most metallic character?
-
If and determine AB. 5 A 3. -1 2 3 2 -1
-
Describe the differences between a general partnership and a limited partnership. Is a general partnership an appropriate form of ownership for two people pooling their resources to start a...
-
Many people who start businesses do not have prior business experience. This is often a worry to people who fit this description. They know that people with experience in accounting, finance, and...
-
What is the purpose of brand management?
-
According to the chapter, prior entrepreneurial experience, relevant industry experience, and networking are attributes that strengthen a persons chances of launching a successful venture. Think...
-
Assume you are opening a restaurant near the college or university you attend and have decided to name it Campus Burger, Wraps, Fries, and Shakes. Based on the location of the college or university,...
-
According to the National Marine Fisheries Service, the current landings in millions of pounds of fish by U.S. fleets are almost double what they were in the 1970s. In other words, fishing has not...
-
In a large midwestern university, 30% of the students live in apartments. If 200 students are randomly selected, find the probability that the number of them living in apartments will be between 55...
-
GAAP requires the statement of cash flows be presented when financial statements are prepared. (a) Explain the purposes of the statement of cash flows. (b) List and describe the three categories of...
-
Brockman Guitar Company is in the business of manufacturing top-quality, steel-string folk guitars. In recent years the company has experienced working capital problems resulting from the procurement...
-
The financial statements of P&G are presented in Appendix 5B or can be accessed at the books companion website, www.wiley.com/college/kieso. Refer to P&Gs financial statements and the accompanying...
-
Information Security:Review the description and the python code for this password generator program and address the following: How does the application currently address Confidentiality,...
-
The enthalpy change when a strong acid is neutralized by strong base is -54.503 kJ/mol. If 140.328 mL of 0.416 M HI at 25.58C is mixed with 163.678 mL of 0.511 M NaOH at 25.58C, what is the maximum...
-
Find the after tax cash flows from operations for Year 1. Would the tax liability be higher or lower with a 6% interest-only loan? What is the after-tax internal rate of return on this investment?...

Study smarter with the SolutionInn App


