Refer to the periodic table inside the front cover of this textbook. State the mass of Avogadros
Question:
Refer to the periodic table inside the front cover of this textbook. State the mass of Avogadro’s number of atoms for each of the following metals.
(a) Silver
(b) Mercury
Periodic Table:
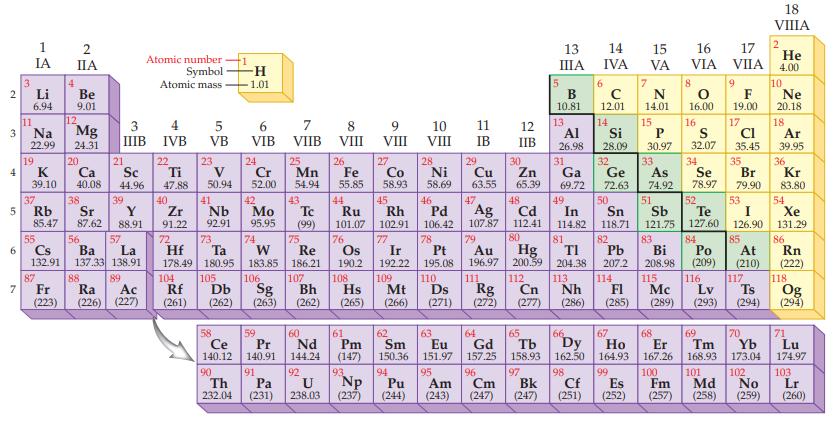
Transcribed Image Text:
2 3 4 5 6 7 3 11 1 IA Li 6.94 Na 22.99 19 37 4 2 IIA 87 12 Fr (223) Be 9.01 Mg 24.31 20 K Ca Sc 39.10 40.08 44.96 38 21 Rb Sr Y 85.47 87.62 88.91 3 4 IIIB IVB 55 56 57 Cs Ba La 132.91 137.33 138.91 88 39 89 Atomic number Symbol Atomic mass Ra Ac (226) (227) 22 Ti 47.88 40 Zr 91.22 5 VB 23 50.94 41 Nb 92.91 -H -1.01 104 105 ᎠᏏ Rf (261) (262) 6 VIB 24 Cr 52.00 42 Mo 95.95 74 106 Sg (263) 72 73 W Re Hf Ta 178.49 180.95 183.85 186.21 59 58 Pr Ce 140.12 90 Th 232.04 (231) 140.91 91 Pa 7 VIIB 25 Mn 54.94 43 Tc (99) 75 60 Nd 144.24 92 8 VIII U 238.03 26 Fe 55.85 44 77 107 Ir 192.22 109 Bh Hs Mt (262) (265) (266) 108 76 Os 190.2 Ru Rh 101.07 102.91 61 Pm (147) 9 VIII 93 27 Np (237) Co 58.93 45 10 VIII 28 Ni 58.69 63 62 Sm Eu 150.36 151.97 94 95 Am Pu (244) (243) 11 IB 29 Cu 63.55 47 13 IIIA 64 65 Gd Tb 157,25 158.93 96 97 Cm Bk (247) (247) 5 16 15 12 Al Si IIB 26.98 28.09 32 30 31 46 52 53 Ag P S 30.97 32.07 33 34 Zn Ga Ge As Se Br 65.39 69.72 72.63 74.92 78.97 79.90 48 49 50 51 Cd In Sn Sb Te I 107.87 112.41 114.82 118.71 121.75 127.60 126.90 80 81 82 Au Pb 196.97 200.59 204.38 207.2 110. 111 112 113 114 Ds Rg Cn Nh FI (271) (272) (277) (286) (285) Pd 106.42 78 79 Pt 195.08 Hg ΤΙ B 10.81 13 14 IVA 6 с 12.01 14 7 15 VA N 14.01 16 17 He VIA VIIA 4.00 8 18 VIIIA 9 10 F Ne 16.00 19.00 20.18 2 17 18 Cl Ar 35.45 39.95 35 36 Kr 83.80 54 Xe 131.29 86 83 84 85 Bi Po At 208.98 (209) (210) 116 117 115 Mc Lv Ts (289) (293) (294) Rn (222) 118 Og (294) 66 70 71 174.97 67 68 69 Dy Ho Er Tm Yb Lu 162.50 164.93 167.26 168.93 173.04 98 99 100 101 102 Cf Es Fm Md (251) (252) (257) (258) No (259) 103 Lr (260)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
The atomic mass of each element is listed below its symbol in the periodic table The mass of Avogadr...View the full answer

Answered By

Robert Mbae
I have been a professional custom essay writer for the last three years. Over that period of time, I have come to learn the value of focusing on the needs of the clients above everything else. With this knowledge, I have worked hard to become an acclaimed writer that can be trusted by the customers to handle the most important custom essays. I have the necessary educational background to handle projects up to the Ph.D. level. Among the types of projects that I've done, I can handle everything within Dissertations, Project Proposals, Research Papers, Term Papers, Essays, Annotated Bibliographies, and Literature Reviews, among others.
Concerning academic integrity, I assure you that you will receive my full and undivided attention through to the completion of every essay writing task. Additionally, I am able and willing to produce 100% custom writings with a guarantee of 0% plagiarism. With my substantial experience, I am conversant with all citation styles ranging from APA, MLA, Harvard, Chicago-Turabian, and their corresponding formatting. With all this in mind, I take it as my obligation to read and understand your instructions, which reflect on the quality of work that I deliver. In my paper writing services, I give value to every single essay order. Besides, whenever I agree to do your order, it means that I have read and reread your instructions and ensured that I have understood and interpreted them accordingly.
Communication is an essential part of a healthy working relationship. Therefore, I ensure that I provide the client with drafts way long before the deadline so that the customer can review the paper and comment. Upon completion of the paper writing service, the client has the time and right to review it and request any adjustments before releasing the payment.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Lucia Company has set the following standard cost per unit for direct materials and direct labor. Direct materials (15 pounds @ $5 per pound) Direct labor (3 hours @ $15 per hour) During May the...
-
Refer to the periodic table (Figure 2.15 or inside front cover) and answer the following questions. a. What Group VIA element is a metalloid? b. What is the Group III A element in Period 3? Figure...
-
Refer to the periodic table (Figure 2.15 or inside front cover) and answer the following questions. a. What Group VA element is a metal? b. What is the Group IIA element in Period 3? Figure 2.15...
-
A health care facility in a metropolitan area is interested in the efficiency of its laboratory turnaround time. Based on data collected over last year, the mean turn around time was found to be 55...
-
Kroger Co.??s 2009 financial statements contained the following data (in millions). InstructionsCompute these values:(a) Working capital. (b) Currentratio. Current assets Total assets Current...
-
Two couples act on the beam. If the resultant couple is to be zero, determine the magnitudes of P and F, and the distance d between A and B. 60 F 0.2 m A d PA 30 B 1m 300 N 2 m 500 N
-
A negatively charged particle is at rest in a region where a uniform magnetic field points in the positive \(x\) direction and a uniform electric field points in the positive \(y\) direction. What is...
-
Ortiz Company is able to produce two products, G and B, with the same machine in its factory. The following information is available. The company presently operates the machine for a single...
-
6. 7. 8. If one of the diameters of the circle x + y-2x-6y+6=0 is a chord of another circle 'C', whose centre is at (2, 1), then its radius is Let Bi (i = 1, 2, 3) be three independent events in a...
-
Balance each of the following combustion reactions by inspection. (a) CH 4 O(l) + O 2 (g) CO 2 (g) + H 2 O(g) (b) C 3 H 8 O(l) + O 2 (g) CO 2 (g) + H 2 O(g).
-
Refer to the periodic table and state the mass of Avogadros number of atoms for each of the following nonmetals. (a) Sulfur (b) Helium. Periodic Table: 19 2 3 4 5 6 7 3 11 1 IA Li 6.94 Na 22.99 19 37...
-
A large hardware store collects data about what its customers buy and stores these data in a data warehouse. If you were the store's buyer for lawn equipment, what would you want to know from the...
-
Forty-eight cubic meters of sewage sludge containing 3.5% solids by mass is fed daily into an anaerobic digester. The solids in the sludge are 68% organic and 32% inert by mass. Some of the organic...
-
You have gathered the following information about the returns on two stocks: A and B. Statistics / Stock A B Expected Return 12% 5% Standard Deviation 24% 19% The correlation between the two stocks...
-
Does the Illinois law about interpreting insurance contracts, exclusionary clauses, and experimental treatments correct as a policy matter? Does it go too far in protecting insureds, get it just...
-
If you open a brokerage account and through that account buy shares of stock in the Apple Corporation on the open market, what type of security are you buying?
-
What is the importance of financial management in a public and non-profit organization? What are the most common techniques used to manage different types of risks? Do you have any work experience in...
-
Taylors is a popular restaurant that offers customers a large dining room and comfortable bar area. Taylor Henry, the owner and manager of the restaurant, has seen the number of patrons increase...
-
The domain of the variable in the expression x 3/x + 4 is________.
-
Change in PrincipleInventory Methods Whitman Company began operations on January 1, 2008, and uses the average cost method of pricing inventory. Management is contemplating a change in inventory...
-
Accounting Change Ramirez Co. decides at the beginning of 2010 to adopt the FIFO method of inventory valuation. Ramirez had used the LIFO method for financial reporting since its inception on January...
-
Accounting Change Linden Company started operations on January 1, 2006, and has used the FIFO method of inventory valuation since its inception. In 2012, it decides to switch to the average cost...
-
Car Crash Investigation Background information: A collision occurred involving two vehicles on Route 28 N. The speed limit in this zone is 45 mph. A 2011 Honda Odyssey minivan was stopped at the...
-
1. A thin film is laid over a glass pane as shown. White light is incident on the film, coming straight in. At a point where the light is incident on the film, it appears green ( = 525 nm). Find (a)...
-
(20%) The output of an argon ion laser can consist of a number of modes of frequency that match the cavity resonance condition and are within the gain bandwidth of the lasing transition. Assume the...

Study smarter with the SolutionInn App


