Silicon is the second most abundant element in Earths crust. Calculate the atomic mass of silicon given
Question:
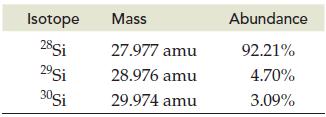
Silicon is the second most abundant element in Earth’s crust. Calculate the atomic mass of silicon given the following data for its three natural isotopes:
Transcribed Image Text:
Isotope 28Si 29Si 30Si Mass 27.977 amu 28.976 amu 29.974 amu Abundance 92.21% 4.70% 3.09%
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
We can find the atomic mass of silicon as follows The av...View the full answer

Answered By

Nazrin Ziad
I am a post graduate in Zoology with specialization in Entomology.I also have a Bachelor degree in Education.I posess more than 10 years of teaching as well as tutoring experience.I have done a project on histopathological analysis on alcohol treated liver of Albino Mice.
I can deal with every field under Biology from basic to advanced level.I can also guide you for your project works related to biological subjects other than tutoring.You can also seek my help for cracking competitive exams with biology as one of the subjects.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
The thin outer layer of Earth, called the crust, contains only 0.50 percent of Earth's total mass and yet is the source of almost all the elements (the atmosphere provides elements such as oxygen,...
-
Although He is the second most abundant element in the universe, it is very rare on the earth. Why?
-
Gerta is 30 years old and advises her financial planner that she wants to retire in 35 years. The financial planner and Gerta determine that Gerta will need to receive beginning of the month payments...
-
Use f(x) and g(x) to find a formula for each expression. Identify its domain. (a) (f + g)(x) (c) (fg)(x) (b) (f- g)(x) (d) (f/g)(x)
-
Factors Affecting Financial Condition. The transmittal letter from the chief financial officer to the mayor and city council of Detroit that accompanies the City of Detroits Comprehensive Annual...
-
General Ship Building Company General Ship Building has a contract with the Department of the Navy to build three new aircraft carriers over the next five years. During the construction of the first...
-
An object moves with constant velocity. What can you say about the work done on a system that includes only this object?
-
Baker Street Stereo is a catalogue ordering operation. The company maintains an ordering staff of 30 telephone operators, who take orders from customers. Management wants to determine the proportion...
-
What are the decision board of direcotrs make at a medical center?
-
Which subatomic particle has a relative charge of +1 and a mass of 1 amu? (a) Alpha (b) Electron (c) Neutron (d) Proton (e) None of the above.
-
State three scientists whose work Dalton used to support the atomic theory.
-
Project Evaluation. The following table presents sales forecasts for Golden Gelt Giftware. The unit price is $40. The unit cost of the giftware is $25. YearUnit Sales 1...
-
Mr. Richards is the department manager for speech pathology services at ABC Hospital. The average productivity per visit at the department is 0.75 hours per procedure. Questions 1. Based on one FTE...
-
Explain the potential far-reaching impact of the NLRB ruling on nursing supervisors. Will this ruling have a chilling effect on nursing unions?
-
Mr. Jones and Ms. Meyers were employed by Lester & Meyer, PLLC, the only law firm in a small town in Oregon. After working on an employment class-action suit under FLSA for several years, Mr. Jones...
-
What are the various elements that compose nurse workload?
-
XYZ Hospital is a community hospital that is not part of a larger healthcare system. This acute care hospital has 160 beds and 348 employees. Benefits and employee pay account for 30 percent of the...
-
Plaintiff Central Hudson Gas and Electric Corporation filed an action against Public Service Commission of New York to challenge the constitutionality of a regulation that completely banned...
-
Why is a help desk and production support critical to system implementations? Discuss its interrelationship with the problem management and reporting system.
-
How does accounting help the capital allocation process?
-
What are some of the major challenges facing the accounting profession?
-
What are the major objectives of financial reporting?
-
Financial Information: On 1 April 2023, the opening balance of the provision for long service leave was $75,000. For the year ended 31 March 2024, Best Sanji Ltd recognised $300,000 for its long...
-
The following table describes the real GDP and population of a fictitious country in 2009 and 2010. Year PIB real Population 2009 $10 billion 1.0 million 2010 $12 billion 1.1 million Instructions:...
-
Analysis of CW's report show that bribery is the most common form of corruption at a local level, followed by procurement and employee expenditure. abuse of power, embezzlement of funds. evaluate the...

Study smarter with the SolutionInn App


