Write a balanced net ionic equation for each of the following acidbase reactions. Refer to Table 14.5
Question:
Write a balanced net ionic equation for each of the following acid–base reactions. Refer to Table 14.5 and Appendix D for electrolyte information.
(a) HCl(aq) + KOH(aq) → KCl(aq) + H2O(l)
(b) HC2H3O2(aq) + Ca(OH)2(aq) → Ca(C2H3O2)2(aq) + H2O(l)
Table 14.5
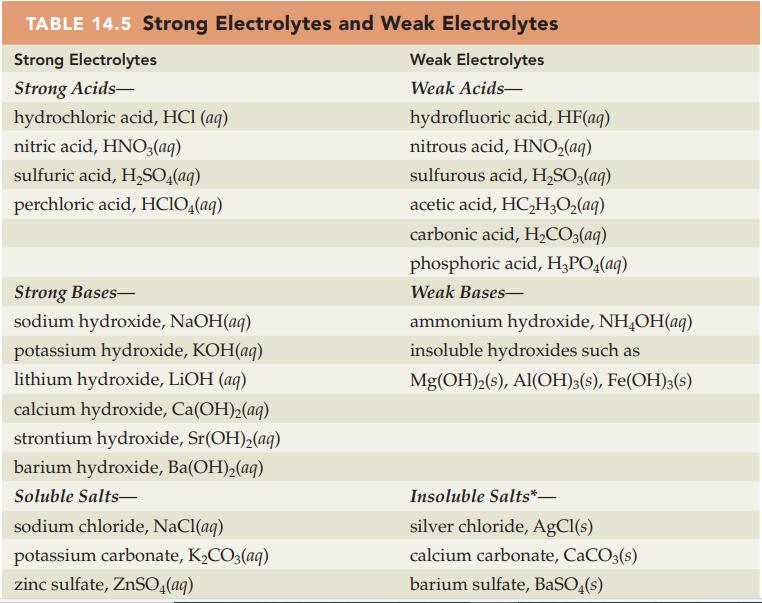
Transcribed Image Text:
TABLE 14.5 Strong Electrolytes and Weak Electrolytes Strong Electrolytes Weak Electrolytes Strong Acids- Weak Acids- hydrochloric acid, HCl (aq) nitric acid, HNO3(aq) sulfuric acid, H₂SO4(aq) perchloric acid, HClO4(aq) Strong Bases- sodium hydroxide, NaOH(aq) potassium hydroxide, KOH(aq) lithium hydroxide, LiOH (aq) calcium hydroxide, Ca(OH)₂(aq) strontium hydroxide, Sr(OH)₂(aq) barium hydroxide, Ba(OH)₂(aq) Soluble Salts- sodium chloride, NaCl(aq) potassium carbonate, K₂CO3(aq) zinc sulfate, ZnSO4(aq) hydrofluoric acid, HF(aq) nitrous acid, HNO₂(aq) sulfurous acid, H₂SO3(aq) acetic acid, HC₂H₂O₂(aq) carbonic acid, H₂CO3(aq) phosphoric acid, H₂PO4(aq) Weak Bases- ammonium hydroxide, NH₂OH(aq) insoluble hydroxides such as Mg(OH)2(s), Al(OH)3(s), Fe(OH)3(s) Insoluble Salts*- silver chloride, AgCl(s) calcium carbonate, CaCO3(s) barium sulfate, BaSO4(s)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
a H aq OH aq H ...View the full answer

Answered By

Shem Ongek
I am a professional who has the highest levels of self-motivation. Additionally, I am always angled at ensuring that my clients get the best of the quality work possible within the deadline. Additionally, I write high quality business papers, generate quality feedback with more focus being on the accounting analysis. I additionally have helped various students here in the past with their research papers which made them move from the C grade to an A-grade. You can trust me 100% with your work and for sure I will handle your papers as if it were my assignment. That is the kind of professionalism that I swore to operate within. I think when rating the quality of my work, 98% of the students I work for always come back with more work which therefore makes me to be just the right person to handle your paper.
4.80+
174+ Reviews
426+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Write a balanced net ionic equation for each of the following reactions: (a) Dilute nitric acid reacts with zinc metal with formation of nitrous oxide. (b) Concentrated nitric acid reacts with sulfur...
-
Write a balanced net ionic equation for each of the following acidbase reactions. Refer to Table 14.5 and Appendix D for electrolyte information. (a) HF(aq) + Li 2 CO 3 (aq) LiF(aq) + H 2 O(l) + CO...
-
Write a balanced net ionic equation for each of the following solution reactions. Refer to Table 14.5 and Appendix D for electrolyte information. (a) Zn(NO 3 ) 2 (aq) + NaOH(aq) Zn(OH) 2 (s) + NaNO...
-
Which of the following statements about close buyer-seller relationships in business markets is FALSE? Long-term commitments on larger order quantities often cause the supplier to raise its selling...
-
Describe the effect on a call options price that results from an increase in each of the following factors: (1) Stock price, (2) Strike price, (3) Time to expiration, (4) Risk-free rate, and (5)...
-
In Exercises determine whether the statement is true or false. If it is false, explain why or give an example that shows it is false. If is continuous on [0, ) and converges. lim f(x) = 0, then fo...
-
Boswell manufactures high-quality speakers. Suppose Boswell is considering spending the following amounts on a new quality program: Boswell expects this quality program to save costs as follows: It...
-
The LP relationships that follow were formulated by Richard Martin at the Long Beach Chemical Company. Which ones are invalid for use in a linear programming problem, and why? Maximize = 6X1 + 12...
-
11. The current in a metallic conductor is plotted against voltage at two different temperatures T and T2. Which is correct :- Current 2 (1) T Voltage (2) T
-
List the four steps for writing a balanced net ionic equation.
-
Write the following salts in either the ionized or the nonionized form to best represent an aqueous solution. (a) AlPO 4 (s) (b) Co(C 2 H 3 O 2 ) 3 (aq) (c) MnSO 4 (aq) (d) PbSO 4 (s).
-
The concentration of atmospheric CO2 has been increasing for many decades. If this trend continues, how might it affect the relative abundance of C3 and C4 plants?
-
What are the pros and cons of buffers or redundant resources from a risk perspective as well as from an efficiency perspective?
-
Enumerate and briefly describe three typical tools and methods that can be used for risk identification.
-
Your company has long relied on a single supplier when procuring a key component for your best-selling product. With product sales still increasing, your top management is increasingly worried about...
-
How does collaborative risk management differ from company-centric risk management?
-
Easyrider Inc. is a Germany-based manufacturer of motorcycles. Easyriders reputa-tion is mostly built on enduro bikes in the high-priced market segment. Easyrider has decided to enter the promising...
-
A Focus on Business Practice box in this chapter lists many companies for which process costing systems are appropriate. Access the websites of at least two of these companies. Find as much...
-
You are thinking of investing in one of two companies. In one annual report, the auditors opinion states that the financial statements were prepared in accordance with generally accepted accounting...
-
Make versus buy, ethics. (CMA, adapted) Lynn Hart is a management accountant at Paibec Corporation. Paibec is under intense cost competition. Hart has been asked to evaluate whether Paibec should...
-
Product mix, constrained resource. Taylor Furniture produces and sells specialty mattresses. Production is a machine-intensive process. Taylors variable costs are direct material costs, variable...
-
Normal and abnormal spoilage in units. The following data, in physical units, describe a grinding process for January: Inspection occurs at the 100% completion stage. Normal spoilage is 5% of the...
-
Soft Touch Company sells leather furniture. The following schedule relates to the company's inventory for the month of April: Cost Sales April 1 Beginning inventory 75 units $44,925 3 Purchase 50...
-
Critically discuss some ethical issues that Tom Ford might encounter in advertising its products to consumers. Your response should describe how these ethical issues might be perceived by various...
-
Watch this video: Nursing simulation scenario: Managing incivility Review this document, which explains the DESC Communication Model Download DESC Communication Model. Discuss ways to share this...

Study smarter with the SolutionInn App


