Reaction rates inside a spherical isothermal porous catalyst particle depend on the rate of diffusion and the
Question:
Reaction rates inside a spherical isothermal porous catalyst particle depend on the rate of diffusion and the kinetics. When the reaction is fast compared to the diffusion, e.g.
at higher temperatures, the reactants will be consumed near the surface and not all the catalyst will be effectively used. The ratio of the actual reaction rate throughout the particle to the reaction rate without any diffusion limitation is a good measure of how effectively the catalyst is used. This is called the effectivenesss factor, η. Consider calculating the effectiveness factor for a spherical catalyst particle for a first-order reaction at the conditions specified below. The governing equation and boundary conditions are given in the dimensionless form, and a first-order reaction is assumed:![dC 2 dC + r dr dr C(1) = 1, dC = 0, dr r=0 r = [0, 1]. - $C = 0, (6.42)](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/2/0/0/4696557001595a0a1700200469138.jpg)
Here the dimensionless variables are concentration c, radius r, and![]() is the Thiele modulus, a dimensionless number used to describe the relation between the reaction and diffusion rates. In this ODE-BVP, the first boundary condition specifies the concentration at the catalyst surface, and the second boundary condition specifies no flux at the center of the particle. In order to use the shooting method, the BVP is rewritten using dC/dr = w, which gives a system of first-order ODEs,
is the Thiele modulus, a dimensionless number used to describe the relation between the reaction and diffusion rates. In this ODE-BVP, the first boundary condition specifies the concentration at the catalyst surface, and the second boundary condition specifies no flux at the center of the particle. In order to use the shooting method, the BVP is rewritten using dC/dr = w, which gives a system of first-order ODEs,
At this point, ordinary ODE-IVP solvers can be used to solve the problem, but, due to the lack of boundary conditions at the beginning of the interval, i.e. at r = 0, the C(r = 0) value needs to be guessed. When a converged solution is obtained, i.e. when
C(r = 1) = 1, the effectiveness factor can be calculated using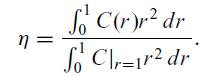
Step by Step Answer:

Mathematical Modeling In Chemical Engineering
ISBN: 9781107049697
1st Edition
Authors: Anders Rasmuson, Bengt Andersson, Louise Olsson, Ronnie Andersson





