The probability that a molecule in a gas will have a speed v is proportional to the
Question:
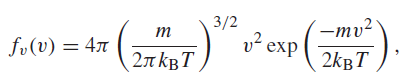
where m is the mass of the molecule, kB is Boltzmann€™s constant, and T is the temperature on the Kelvin scale. The most probable speed is the speed for which this function is at a maximum. Find the expression for the most probable speed and find its value for nitrogen molecules at T = 298 K. Remember to use the mass of a molecule, not the mass of a mole.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





