Ethyl vanillin is used in food, cosmetics, and pharmaceuticals for its vanilla-like scent. The article Determination and
Question:
Ethyl vanillin is used in food, cosmetics, and pharmaceuticals for its vanilla-like scent. The article “Determination and Cor Correlation of Ethyl Vanillin Solubility in Different Binary Solvents at Temperatures from 273.15 to 313.15 K” (J. Chem. Engr. Data 2017: 1788–1796) reported an experiment to determine y = ethyl vanillin solubility (mole fraction) as a function of x1 = initial mole fraction of the chemical propan-2-one in the solvent mixture and x2 = temperature (°K). The experiment was run at seven x1 and nine x2 values. The accompanying table shows the response, y, at each combination.
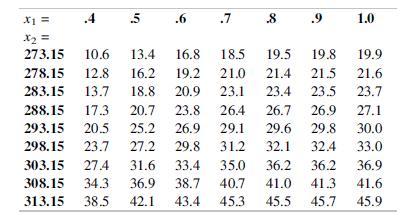
a. Create scatterplots of y versus x1 and y versus x2. Does it appear the predictors are linearly related to y, or would quadratic terms be appropriate?
b. Would a scatterplot of x1 versus x2 indicate whether an interaction term might be suitable? Why or why not?
c. Perform a regression using the complete second-order model. Based on a residual analysis, does it appear that the model assumptions are satisfied?
d. Test various hypotheses to determine which term(s) should be retained in the model.
Step by Step Answer:

Modern Mathematical Statistics With Applications
ISBN: 9783030551551
3rd Edition
Authors: Jay L. Devore, Kenneth N. Berk, Matthew A. Carlton





