Use the partition functions in Equations 1.98, 1.99, and 1.100 to find the translational, rotational, and vibrational
Question:
Use the partition functions in Equations 1.98, 1.99, and 1.100 to find the translational, rotational, and vibrational contributions to the average energy of a diatomic molecule. Compare each result to the prediction of the equipartition theorem, which states that in the classical limit each degree of freedom contributes 12(1/2)kBT to the average energy.
Equations 1.98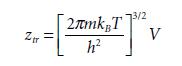
Equations 1.99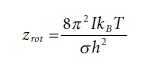
Equations 1.100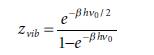
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





