When 1,2-dimethylcyclohexene is allowed to react with hydrogen in the presence of a platinum catalyst, the product
Question:
When 1,2-dimethylcyclohexene is allowed to react with hydrogen in the presence of a platinum catalyst, the product of the reaction is a cycloalkane that has a melting point of −50 °C and a boiling point of 130 °C (at 760 torr).
(a) What is the structure of the product of this reaction?
(b) Consult an appropriate resource (such as the web or a CRC handbook) and tell which stereoisomer it is.
(c) What does this experiment suggest about the mode of addition of hydrogen to the double bond?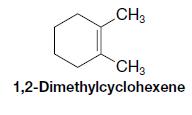
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Organic Chemistry
ISBN: 978-1118875766
12th Edition
Authors: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Question Posted:





