A compound A (C 11 H 14 O 3 ) is insoluble in base and gives an
Question:
A compound A (C11H14O3) is insoluble in base and gives an isomeric compound B when heated strongly. Compound B gives a sodium salt when treated with NaOH.
Treatment of the sodium salt of B with dimethyl sulfate gives a new compound C (C12H16O3) that is identical in all respects to a natural product elemicin. Ozonolysis of elemicin followed by oxidation gives the carboxylic acid D. Propose structures for compounds A, B, and C.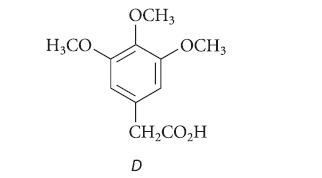
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





