A DielsAlder reaction of 2,5-dimethylfuran and maleic anhydride gives a compound A that undergoes acidcatalyzed dehydration to
Question:
A Diels–Alder reaction of 2,5-dimethylfuran and maleic anhydride gives a compound A that undergoes acidcatalyzed dehydration to give 3,6-dimethyl phthalic anhydride (see Fig. P16.65).
(a) Deduce the structure of compound A.
(b) Give a curved-arrow mechanism for the conversion of A into 3,6-dimethylphthalic anhydride.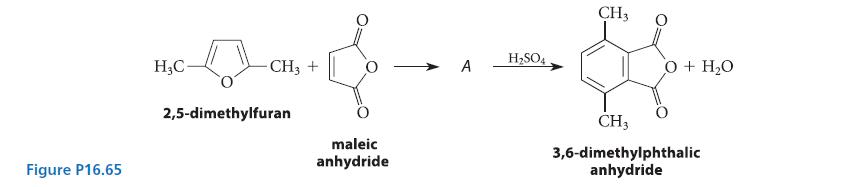
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





