(a) Explain why compound A has a UV spectrum with considerably greater max values and intensities...
Question:
(a) Explain why compound A has a UV spectrum with considerably greater λmax values and intensities than are observed for ethylbenzene.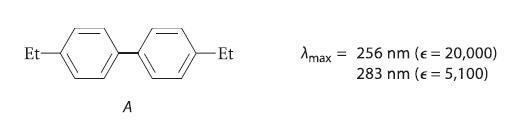
(b) In view of your answer to part (a), explain why the UV spectra of compounds B and C are virtually identical.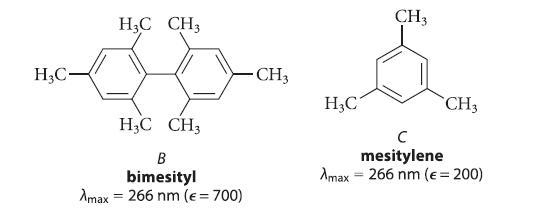
Transcribed Image Text:
Et A Et Amax 256 nm (e = 20,000) 283 nm (e 5,100) =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
a The two benzene rings are conjugated in compound A because there are more bonds in conjugation the ...View the full answer

Answered By

Nimlord Kingori
2023 is my 7th year in academic writing, I have grown to be that tutor who will help raise your grade and better your GPA. At a fraction of the cost on other sites, I will work on your assignment by taking it as mine. I give it all the attention it deserves and ensures you get the grade that I promise. I am well versed in business-related subjects, information technology, Nursing, history, poetry, and statistics. Some software's that I have access to are SPSS and NVIVO. I kindly encourage you to try me; I may be all that you have been seeking, thank you.
4.90+
360+ Reviews
1070+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
In a national poll of 1039 U.S. adults, conducted November 7-10, 2013, Gallup asked "Roughly how much money do you think you personally will spend on Christmas gifts this year?". The data provided on...
-
In the article Sweetening StatisticsWhat M&Ms Can Teach Us (Minitab Inc., August 2008), M. Paret and E. Martz discussed several statistical analyses that they performed on bags of M&Ms. The authors...
-
In a poll of 1009 U.S. adults of age 18 years and older, conducted December 47, 2008, Gallup asked Roughly how much money do you think you personally will spend on Christmas gifts this year?. The...
-
How are direct and indirect materials costs distinguished?
-
Relating net income to balance sheet changes Magyar T1ekom (Magyar), a Hungarian telecommunications company, reported the following balance sheet information for the years ended December 31, 2006 and...
-
In its 2008 annual report, Nike, Inc., the athletic sportswear company, provided the following data about its current and deferred income tax provisions (in millions): 2008 Current income taxes due...
-
In a baseball game, a batter hits the \(0.150-\mathrm{kg}\) ball straight back at the pitcher at \(180 \mathrm{~km} / \mathrm{h}\). If the ball is traveling at \(160 \mathrm{~km} / \mathrm{h}\) just...
-
Selected pre-adjustment account balances and adjusting information of Sunset Cosmetics Inc. for the year ended December 31, 2011, are as follows: Retained Earnings, January 1, 2011 . . . . . . . . ....
-
What do the following abbreviations mean? a) I/O: input/output devices b) CPU: central processing unit or processor c) RAM: random access memory or main memory d) GHz: Gigahertz billions of cycles...
-
A benzene derivative known to be a methyl ether with the formula C 7 H 6 OCl 2 has five lines in its proton-decoupled 13 C NMR spectrum. Propose two possible structures for this compound that fit...
-
Predict the approximate boiling point of (a) Ethylbenzene (b) Propylbenzene (c) P-xylene
-
5/8 3/4 Perform the indicated operation by hand.
-
What are two types of control frequently used on projects?
-
The sponsor of a large multiphased project you are managing suddenly decides to terminate the project early. How do you respond? How and when do you notify your team members?
-
Determine what type of contract you would use for this work and explain why.
-
You are the project manager in charge of renovating a large apartment building, and your team has decided to outsource the installation of a new septic system. Do you put out an RFQ or RFP to...
-
What five aspects of project success are evaluated in the balanced scorecard approach?
-
Does age make a difference in the amount of savings a worker feels is needed to be secure at retirement? A study by CommSciences for Transamerica Asset Management found that .24 of workers in the...
-
The text defined intrinsic value as the value of an asset given a hypothetically complete understanding of the assets investment characteristics. Discuss why hypothetically is included in the...
-
Rank the following compounds in order of increasing reactivity (least reactive first) in an SN1 solvolysis reaction in aqueous acetone. Explain your answers. (The structure of tert-cumyl chloride is...
-
Explain why compound A reacts faster than compound B when they undergo solvolysis in aqueous acetone. CH C-Cl CH3 CH CH
-
A hydrocarbon A, C9H12, is treated with A-bromosuccinimide in CCl4 in the presence of peroxides to give a compound B, C9H11Br. Compound B undergoes rapid solvolysis in aqueous acetone to give an...
-
What three schedules about deferred taxes are disclosed in the footnotes of the financial statements? Note: you may want to review Target's tax note to understand text book on required disclosures
-
You will need to refer to the Document filing and retention policy. Here are the questions you need to answer: How are invoices and supporting documentation filed for audit purposes? What is the...
-
Automated Production Lines (15 points) An automated production line with the following information: Number of stations 24 station Ideal cycle time 0.85 min Average down time/line 9 min Station...

Study smarter with the SolutionInn App


