(a) From the extinction coefficient of isoprene (10,750 M 1 cm 1 ) and its observed absorbance...
Question:
(a) From the extinction coefficient of isoprene (10,750 M–1 cm–1) and its observed absorbance at 222.5 nm (Fig. 15.5), calculate the concentration of isoprene in mol L–1 (assume a 1 cm light path).
(b) From the results of part (a) and Fig. 15.5, calculate the extinction coefficient of isoprene at 235 nm.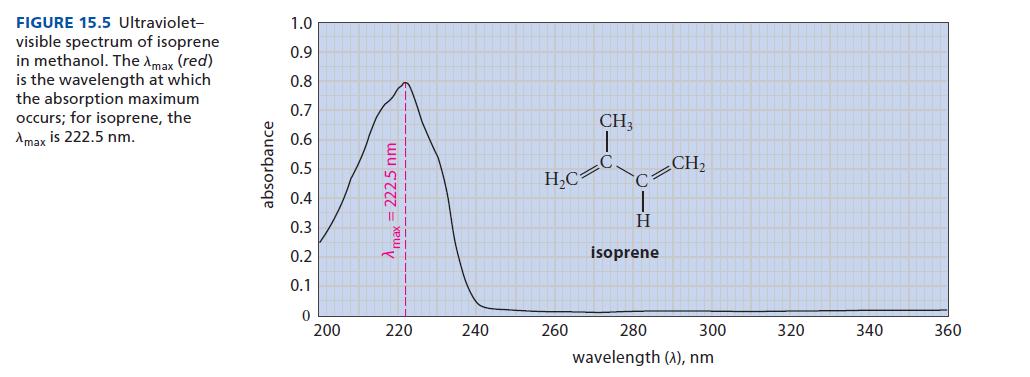
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





