(a) Use the relative bond lengths of the CC and CO bonds to predict which of the...
Question:
(a) Use the relative bond lengths of the C—C and C—O bonds to predict which of the following two equilibria lies farther to the right. (That is, predict which of the two compounds contains more of the conformation with the axial methyl group.)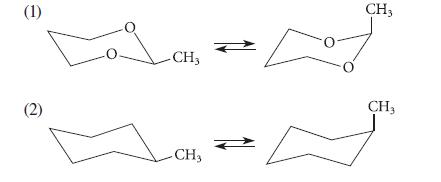
(b) Which one of the following compounds contains the greater amount of gauche conformation for internal rotation about the bond shown? Explain.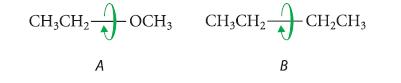
Transcribed Image Text:
(1) (2) -CH3 - CH3 ← CH3 CH₂
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
a Equilibrium 2 contains more of the conformation with the methyl group in an axial position than eq...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
a. Predict the relative bond lengths of the three carbon-oxygen bonds in the carbonate ion (CO23-) b. What would you expect the charge to be on each oxygen atom?
-
Kenny operates a store, where he sells feed and other supplies to farmers. Heather purchases a $20,000 tractor from Kenny and pays Kenny with $18,000 in cash and $2,000 in corn. How much gross income...
-
Name five techniques you can use to ensure that visual aids do not distort graphic information.
-
Which of the costs incurred in completing a business combination should be treated as a reduction of additional paid-in capital?
-
A blood pressure measurement consists of two numbers: the systolic pressure, which is the maximum pressure taken when the heart is contracting, and the diastolic pressure, which is the minimum...
-
Suppose that the market can be described by the following three sources of systematic risk with associated risk premiums. Factor Risk Premium Industrial production (I) ........ 6% Interest rates (R)...
-
WeBWorK 5 - Topics 10 - 12: Problem 4 (1 point) dt, then F'(x) = a) If F(x) = = dt, then 23 b) If F(x) = 2 t dt, then F'(x) = t c) If F(x) = Lo 1 dt, then F'(x) = t x+1 1 c) If F(x) = dt, then F'(x)...
-
Consider the reaction analyzed in Study Problem 3.2 (p. 91), reproduced below. Identify the nucleophilic center, the electrophilic center, and the leaving group in the forward direction. (Dont...
-
Offer an explanation for each of the following observations. (a) Compound A exists mostly in a chair conformation with an equatorial OH group, but compound B prefers a chair conformation with an...
-
Explain why management of a company using IFRS might choose to classify the payment of dividends as an operating activity.
-
A quote from Bloomberg news October 6, 2013: "The yield on 10-year U.S. bonds dropped to a two-month low of 2.58 percent on Oct. 3, after Treasury Secretary Jacob J. Lew said the government won't be...
-
To use Hooke's law and the definitions of stress and strain to calculate unknown values for linear elastic deformation of a rod. A material undergoing uniaxial elastic deformation obeys Hooke's law....
-
Firm A (whose shares were trading at $10 per share at close yesterday) makes an offer (at 9 pm) to buy shares in Firm B (whose shares were trading at $5 per share at close yesterday).. What is the...
-
Description of kids toy that will be supported with marketing activities(product) What pricing strategy will be employed for kids toys during the marketing period and why? (Price) Describe the...
-
You have founded a company to sell thin client computers to the food processing industry for Internet transaction processing. Before investing in your new company, a venture capitalist has asked for...
-
A person must score in the upper 2% of the population on an IQ test to qualify for membership in Mensa, the international high-IQ society. There are 110,000 Mensa members in 100 countries throughout...
-
What is a make-or-buy decision?
-
This alkynes hydration reaction can occur without added Hg2+. Show all the steps in themechanism. H,SO, PhC=CH + H20 PHCCH3
-
Explain which compound has a faster rate of reaction withHCI: b) or or NO2 or
-
The addition of C1 to (E)-2-pentene produces a recemic mixture of (2R,3S)-2,3- dichloropentane and its enantiomers, 2R,3S)-2,3- dichloropentane. (a) Show the structure of the two chloronium ions that...
-
5. Produce the complete input/output table for this circuit (3 pts): R- P- NOT AND Do. NOT NOT
-
Construct a clean and concise ER diagram for the following scenario. List your assumptions and clearly indicate the cardinality mappings in your ER diagram. . A driver identified by driver Sin...
-
Caesar Cipher is an encryption technique. It is a type of substitution cipher in which each letter in the plaintext is replaced by a letter some fixed number of positions along the alphabet. For...

Study smarter with the SolutionInn App


