As shown in the following equation, when (R)-1-deuterio-1-butanamine is diazotized with nitrous acid in water, the alcohol
Question:
As shown in the following equation, when (R)-1-deuterio-1-butanamine is diazotized with nitrous acid in water, the alcohol product formed has the S configuration (D = 2H).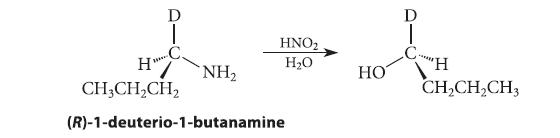
(a) Give the stereochemical configuration of the diazonium ion formed as an intermediate in this reaction. Draw its structure.
(b) What mechanism for reaction of the diazonium ion with water is consistent with the stereochemical result in the preceding equation?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





