Question: Furan is an aromatic compound. Discuss the hybridization of its oxygen and the geometry of its two electron pairs. furan (aromatic)
Furan is an aromatic compound. Discuss the hybridization of its oxygen and the geometry of its two electron pairs.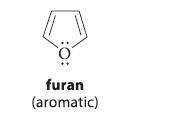
furan (aromatic)
Step by Step Solution
3.54 Rating (157 Votes )
There are 3 Steps involved in it
Given that furan is aromatic it can have 4n 27electrons six electron... View full answer

Get step-by-step solutions from verified subject matter experts


