In each of the following parts, explain why the first compound has a higher boiling point than
Question:
In each of the following parts, explain why the first compound has a higher boiling point than the second, despite a lower molecular mass.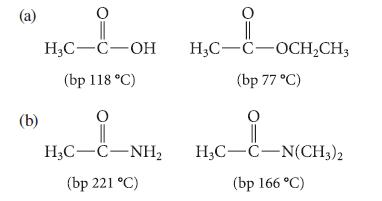
Transcribed Image Text:
(a) (b) H₂C-C-OH (bp 118 °C) O H3C-C-NH₂ (bp 221 °C) H₂C-C-OCH₂CH3 (bp 77 °C) O H3C-C-N(CH3)2 (bp 166 °C)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
a Explanation Acetic acid CH3COOH has higher boiling point 118 C than ethyl acetate CH3COOCH2CH3 bp ...View the full answer

Answered By

Vijesh J
My passion to become a tutor is a lifetime milestone. Being a finance and marketing professional with hands-on experience in wealth management, portfolio management, team handling and actively contributing in promoting the company. Highly talented in managing and educating students in most attractive ways were students get involved. I will always give perfection to my works. Time is the most important for the works and I provide every answer on time without a delay. I will proofread each and every work and will deliver a with more perfection.
4.70+
5+ Reviews
15+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
In each of the following parts. Explain why the first compound has a higher boiling pc-rint than the second, despite a lower molecular mass. (bp 221 C) (bp 166 C)
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Explain why a. H2O has a higher boiling point than CH3OH(65oC). b. H2O has a higher boiling point than NH3(- 33oC). c. H2O has a higher boiling point than HF (20C).
-
Do you agree or not? Specialization of labor and better use of capital goods can initially generate increasing marginal output (returns) for a firm in the production of a good.
-
Was the Calcu-Folio properly classified as a briefcase or a binder?
-
On January 1, 20X3, PURE Products Corporation issued 12,000 shares of its $10 par value stock to acquire the net assets of Light Steel Company. Underlying book value and fair value information for...
-
For a given data set containing 18 points, the assumptions of the linear model are satisfied. The following values are computed: b1 = 5.58 and sb = 4.42. Perform a test of the hypothesis H0 : 1 = 0...
-
The ledger of Lentz Company includes the following unadjusted balances: Prepaid Insurance $3,000, Service Revenue $58,000, and Salaries and Wages Expense $25,000. Adjusting entries are required for...
-
Aram Industries Inc. issues 30-year bonds with an annual coupon rate of 7.5%. If the bond is currently traded at $925, what is its yield to maturity?
-
Without consulting tables, arrange the compounds within each of the following sets in order of increasing boiling point, and give your reasoning. (a) 1-hexanol, 2-pentanol, tert-butyl alcohol (b)...
-
For the following problems, see Table 8.2 for structures and dipole moments. Explain your reasoning in each case. (a) With which one of the following solvents is DMSO not miscible: water, acetone,...
-
In Problems 4374, find the real solutions of each equation. x 6 - 7x 3 - 8 = 0
-
Shipments of Product X from a plant to a wholesaler are made in lots of 1500 units each. The wholesaler's average demand for X is 300 units per week. The lead time from the plant to the wholesaler is...
-
2. For the titration of 250 mL of 0.200 mol L-1 HF(Ka = 6.3 x 10-4) with a solution of 1.00 M NaOH: a) What is the pH at the half-equivalence point? b) What is the pH at the equivalence point?
-
When inputting an answer, round your answer to the nearest 2 decimal places (for VARIANCE round to 4 decimal places). If you need to use a calculated number for further calculations, DO NOT round...
-
What is a common product that the business sells? What are the main costs the business has to incur in order to be able to sell that product? Which of these costs are fixed and which are variable?...
-
Three charges are placed as shown in the figure below. A scale is provided for the distances. Match the numerical values with the appropriate force of interaction. 19=8c a. b. 9=-2C I'm 93=4...
-
Comcast Corporation is the largest cable television company, the second largest Internet service provider, and the fourth largest telephone service provider in the United States. Generally known for...
-
Interest Compounded Annually. When P dollars is invested at interest rate i, compounded annually, for t years, the investment grows to A dollars, where A = P(1 + i) t . Trevor's parents deposit $7800...
-
Name these compounds. Discuss.
-
Explain which compound has the higher melting point.
-
Explain which compound has the higher boiling point. Discuss in detail.
-
In this case, we investigate the effect of wage changes on inflation rate. Such effects can be from the demand side or the supply side. On the supply side, we expect wage increases to increase...
-
Jay-Jay's Clothing produces uniforms for employees from large corporations. The company provides the following information for one of its uniforms: Direct materials standard Direct manufacturing...
-
Deming & Sons manufactures four grades of lubricant, W-10, W-20, W-30, and W-40, from a joint process. Additional information follows: Product Units Produced Sales Value at Split-Off Additional Costs...

Study smarter with the SolutionInn App


