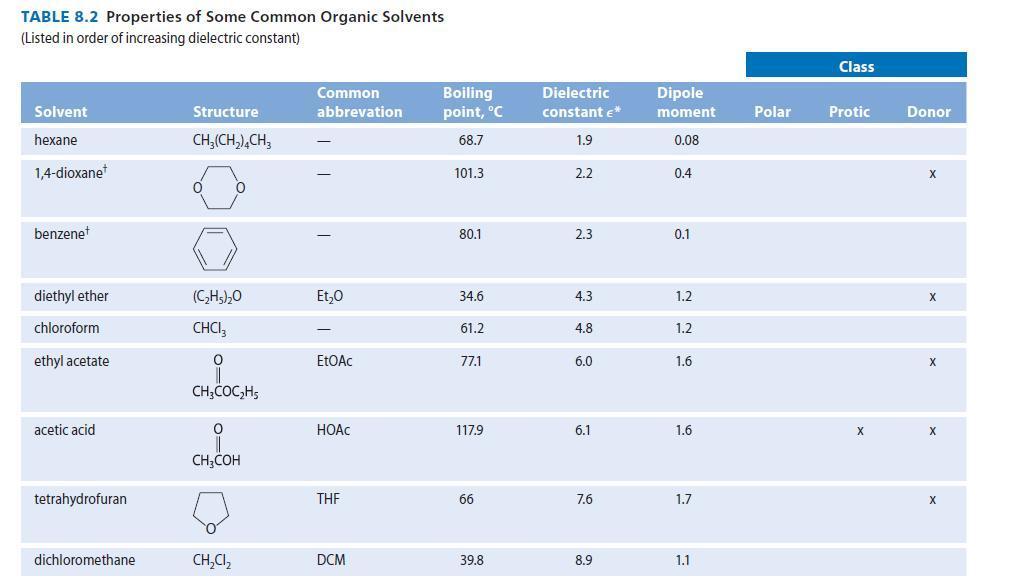
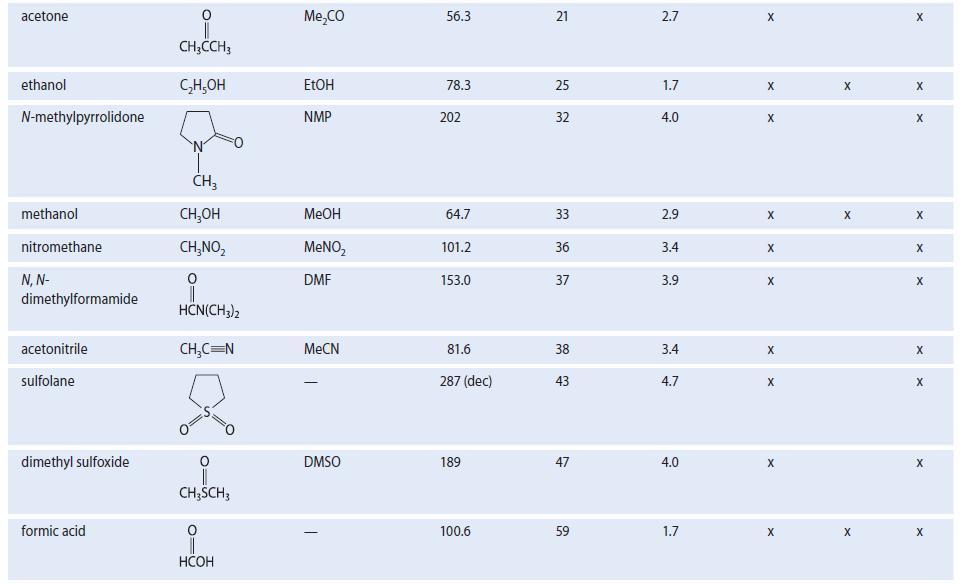
For the following problems, see Table 8.2 for structures and dipole moments. Explain your reasoning in each
Question:
For the following problems, see Table 8.2 for structures and dipole moments. Explain your reasoning in each case.
(a) With which one of the following solvents is DMSO not miscible: water, acetone, hexane, or acetonitrile?
(b) With which one of the following solvents is hexane not miscible: methanol, 1-propanol, diethyl ether, or acetone?

Transcribed Image Text:
TABLE 8.2 Properties of Some Common Organic Solvents (Listed in order of increasing dielectric constant) Solvent hexane 1,4-dioxane benzenet diethyl ether chloroform ethyl acetate acetic acid tetrahydrofuran dichloromethane Structure CH₂(CH₂), CH3 0 (C₂H5)₂0 CHCI 0 CH3COC₂H5 0 CH3COH CH₂Cl₂ Common abbrevation Et₂0 EtOAc HOAC THE DCM Boiling point, °C 68.7 101.3 80.1 34.6 61.2 77.1 117.9 66 39.8 Dielectric constant €* 1.9 2.2 2.3 4.3 4.8 6.0 6.1 7.6 8.9 Dipole moment 0.08 0.4 0.1 1.2 1.2 1.6 1.6 1.7 1.1 Polar Class Protic X Donor X X X X X
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
The ability of solvents to mix with each other miscibility is largely dependent on the similarity of their polarity and ability to engage in hydrogen ...View the full answer

Answered By

ANDREW KIPRUTO
Academic Writing Expert
I have over 7 years of research and application experience. I am trained and licensed to provide expertise in IT information, computer sciences related topics and other units like chemistry, Business, law, biology, biochemistry, and genetics. I'm a network and IT admin with +8 years of experience in all kind of environments.
I can help you in the following areas:
Networking
- Ethernet, Wireless Airmax and 802.11, fiber networks on GPON/GEPON and WDM
- Protocols and IP Services: VLANs, LACP, ACLs, VPNs, OSPF, BGP, RADIUS, PPPoE, DNS, Proxies, SNMP
- Vendors: MikroTik, Ubiquiti, Cisco, Juniper, HP, Dell, DrayTek, SMC, Zyxel, Furukawa Electric, and many more
- Monitoring Systems: PRTG, Zabbix, Whatsup Gold, TheDude, RRDtoo
Always available for new projects! Contact me for any inquiries
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Which would you expect to be the stronger acid? Explain your reasoning in each instance. (a) CH2ClCO2H or CHCl2CO2H (b) CCl3CO2H or CHCl2CO2H (c) CH2FCO2H or CH2BrCO2H (d) CH2FCO2H or CH2FCH2CO2H
-
The file S02_35.xlsx contains (fictional) data from a survey of 500 randomly selected households. a. Indicate the type of data for each of the variables included in the survey. b. For each of the...
-
The auditor uses MUS to select accounts receivables for confirmation. In confirming individual accounts receivable balances, your client's customers reported the exceptions listed below. Required...
-
Lacoste t-shirts come with an average price of $ 120 a piece, at their factory outlet with a std. deviation of $ 17. But at the Seasonal Sale (Discount) outlets of these t- shirts, it was also...
-
The court states that the whole purpose of a letter of credit would be defeated by examining the merits of the underlying contract. What does that mean?
-
The town of Dinsmore passed a bill requiring that all homes be connected to the town sewer system. Baskin Ridge is the only section of town that does not have town sewers. Dinsmore will finance the...
-
Refer to the information in Exercise 24-3 and assume instead that double-declining depreciation is applied. Compute the machines payback period (ignore taxes). (Round the payback period to three...
-
Given the following network with activity times in months, determine the earliest start and finish times, latest start and finish times, and slack for each activity. Indicate the critical path and...
-
__________ are expenses that can be subtracted from total income in order to calculate tax liability. They may include child care expenses and union dues. deductions debentures tax credits dividends...
-
In each of the following parts, explain why the first compound has a higher boiling point than the second, despite a lower molecular mass. (a) (b) HC-C-OH (bp 118 C) O H3C-C-NH (bp 221 C) HC-C-OCHCH3...
-
Give an IUPAC name for each of the following compounds, which may have been isolated from the shoes of a tennis player. Ignore stereochemistry in (a). (a) (b) HO
-
Star \(\alpha\), of mass \(m\), is headed directly towards Star \(\beta\), of mass \(3 m\), with velocity \(v_{0}\) as measured in \(\beta^{\prime} s\) rest frame. (a) What is the velocity of their...
-
To maximize the expected profit from the potential sale, what posted price would you commit to to maximize the expected value from the potential sale of the machine?
-
The figure below represents a backfill behind a smooth vertical retaining wall. Estimate the magnitude and line of action of the lateral active force per meter length of the wall. What would be the...
-
You are the sole shareholder of an S corporation that earns 150,000 of net income. You then decide to pay yourself a salary from the S corporation in the amount of 150,000, reducing the S corporation...
-
Suppose that the demand for classical music concert tickets is negatively sloping and the supply of classical music concert tickets is positively sloping.Classical music lovers persuade Congress to...
-
Which do you consider to be the most important of the 8 millennium development goals and why? Have the MDGs achieved their goals so far? Why or why not?
-
Assume a monopsonistic employer is paying a wage rate of Wm and hiring Qm workers, as indicated in Figure. Bilateral monopoly in the labor market. Now suppose an industrial union is formed that...
-
Interest Compounded Annually. When P dollars is invested at interest rate i, compounded annually, for t years, the investment grows to A dollars, where A = P(1 + i) t . Trevor's parents deposit $7800...
-
Explain whether each compound is soluble in aqueous NaOH, aqueous NaHCO3 both, or neither.
-
Calculate these quantities: (a) The wavelength of light (in centimeters) with a frequency of 9.00 x 1012Hz. (b) The frequency of light with a wavelength of 310nm (c) The energy of light (in kcal/mol...
-
What kind of light has a frequency of 9.00 x 1013Hz?
-
A 2.97 kg particle has a velocity of (3.08 - 4.03 ) m/s. (a) Find its x and y components of momentum. Px Py = = kg.m/s kg.m/s (b) Find the magnitude and direction of its momentum. kg.m/s (clockwise...
-
A rod of length 38.50 cm has linear density (mass per length) given by = 50.0 18.0x where x is the distance from one end, and 1 is measured in grams/meter. (a) What is its mass? 19.25 Your response...
-
File Input/Output Objective: Create a program that allows a user to fill in values for two separate arrays, then saves that information in a separate file. Related SLOs: SLO #1: Use an appropriate...

Study smarter with the SolutionInn App


