Isopentenyl pyrophosphate, the starting material for isoprenoid and steroid biosynthesis (Sec. 17.6B), is formed biosynthetically by the
Question:
Isopentenyl pyrophosphate, the starting material for isoprenoid and steroid biosynthesis (Sec. 17.6B), is formed biosynthetically by the decarboxylation reaction of (R)-phosphomevalonate-5-pyrophosphate catalyzed by the enzyme mevalonate-5-pyrophosphate decarboxylase, shown in Fig. P20.55. When a different starting material is used in which the —CH3 group is replaced with a fluoromethyl (FCH2—) group, the reaction takes place 2500 times more slowly.
(a) Draw a two-step curved-arrow mechanism that includes the structure of the intermediate X. Your mechanism should account for the large effect of fluorination on the rate of the reaction. (Assume acids and bases are available as needed.)
(b) What is the “electron sink” for the decarboxylation reaction?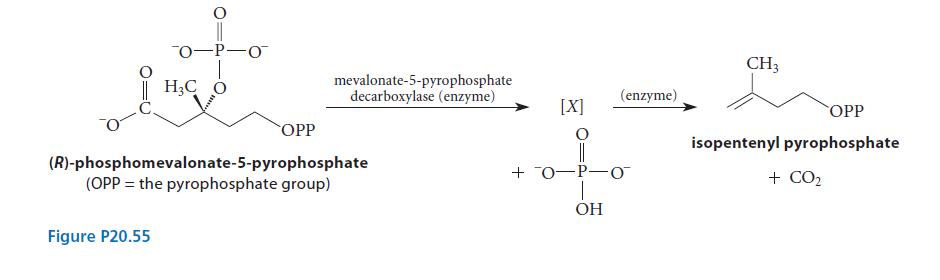
Step by Step Answer:






