Nitration of phenyl acetate (compound A) results in para substitution of the nitro group. However, nitration of
Question:
Nitration of phenyl acetate (compound A) results in para substitution of the nitro group. However, nitration of dimethyl phenyl phosphate (compound B) results in meta substitution of the nitro group. Suggest a reason that the two compounds nitrate in different positions.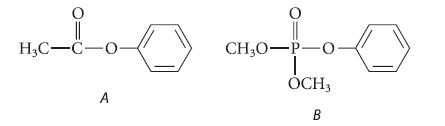
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





