Tell whether each of the following reactions favors reactants or products at equilibrium. (Assume that all reactants
Question:
Tell whether each of the following reactions favors reactants or products at equilibrium. (Assume that all reactants and products are soluble.)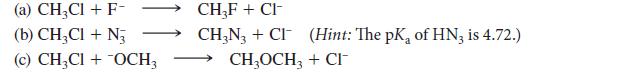
Transcribed Image Text:
(a) CH₂Cl + F- (b) CH3CI + N3 (c) CH₂Cl + OCH3 CH₂F+CI- CH₂N3+CI (Hint: The pK, of HN3 is 4.72.) CH3OCH3 + CI™
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
a Because fluoride ion is a stronger base than chloride ...View the full answer

Answered By

Muqadas Javed
I am a mentor by profession since seven years. I have been teaching on online forums and in universities. Teaching is my passion therefore i always try to find simple solution for complicated problems or task grasp them so that students can easily grasp them.I will provide you very detailed and self explanatory answers and that will help you to get good grade. I have two slogans: quality solution and on time delivery.
4.60+
24+ Reviews
144+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Tell wbether each of the following reactions favors reactants or products at equilibrium. (Assume that all reactants and products are soluble.) (a) CH3CI + I- CH3I + CI- (b) CH3CI + -OCH3 CH3OCH3 +...
-
Write a detailed paper on Trademark Law of the People's Republic of China
-
Assume the market for cat food is perfectly competitive. The cost curves for a typical cat food producer are depicted below. Indicate whether each of the following statements is true or false AND...
-
1) A survey of 200 public universities indicated that the 25th percentile of the yearly tuition cost of the universities was $4600 and the 75th percentile was $7100. The minimum value was $2000, the...
-
Describe the test for determining whether a governmental fund is a major fund. Describe the test for determining whether an enterprise fund is a major fund.
-
Nissan produces a car that sells in Japan for 1.8 million. On September 1, the beginning of the model year, the exchange rate is 150:$1. Consequently, Nissan sets the U.S. sticker price at $12,000....
-
Continuing to focus on evidence associated with the act, concealment, and conversion, use the evidentiary material to continue the examination. In addition, the examiner also starts to think of terms...
-
Forney Company maintains a petty cash fund for small expenditures. The following transactions occurred over a 2-month period. July 1 Established petty cash fund by writing a check on Scranton Bank...
-
Simplify: 6/8p 98p.
-
What substitution and elimination products (if any) might be obtained when each of the following alkyl halides is treated with sodium methoxide in methanol? (a) Trans-1-bromo-3-methylcyclohexane (b)...
-
What product(s) are expected in the ethoxide-promoted b-elimination reaction of each of the following compounds? (a) 2-bromo-2,3-dimethylbutane (b) 1-chloro-1-methylcyclohexane
-
Why is the pricing of services more difficult than the pricing of goods?
-
Assess the significance of telephone screening and use of the Internet in employee selection.
-
Bach Musics most recent annual dividend was $1.50 per share (D 0 = 1.50), and the firms required return is 9%. Find the market value of Bachs shares when: a. Dividends are expected to grow at 10%...
-
Bill Clinton reportedly was paid $15 million to write his book My Life. Suppose the book took three years to write. In the time he spent writing, Clinton could have been paid to make speeches. Given...
-
What are the pros and cons of the three different pairing assignment methods used by airlines.
-
What is the relationship between personal construct theory and the repertory grid?
-
How would the capital-budgeting procedures of a firm that chooses to build differ from those used by a firm that chooses to harvest? Why might they differ?
-
Three forces with magnitudes of 70pounds, 40 pounds, and 60 pounds act on an object at angles of 30, 45, and 135, respectively, with the positive x-axis. Find the direction and magnitude of the...
-
Addition of HBr to a double bond with an ether (?OR) substituent occurs region specifically to give a product in which the ?Br and ?OR are bonded to the same carbon. Draw the two possible carbocation...
-
Alkyl halides can be reduced to alkanes by a radical reaction with tributyltin hydride, (C 4 H 9 ) 3 SnH, in the presence of light (h v ). Propose a radical chain mechanism by which the reaction...
-
Identify the reagents a?c in the following scheme: CH Br CH CH C CH
-
K A used car dealer says that the mean price of a three-year-old sports utility vehicle is $20,000. You suspect this claim is incorrect and find that a random sample of 20 similar vehicles has a mean...
-
Shmueli and Koppius ( 2 0 1 1 ) concluded the roles of predictive analytics in scientific research. Based on the context of online reviews, can you provide an example?
-
If we have an interval width of 5 , and 2 8 is the lowest observed value in our data, what should our starting point be for a grouped frequency table?

Study smarter with the SolutionInn App


