The molecules nitromethane and 2-propanol have roughly the same shape and molecular mass. Liquid 2-propanol contains hydrogen
Question:
The molecules nitromethane and 2-propanol have roughly the same shape and molecular mass.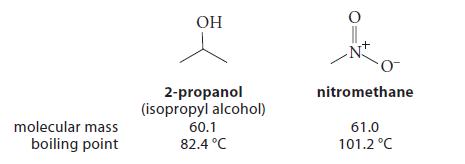
Liquid 2-propanol contains hydrogen bonds, but liquid nitromethane does not. Yet nitromethane has the higher boiling point. Why does nitromethane have such a high boiling point? What physical properties of the two molecules could you look up to support your answer?
Transcribed Image Text:
molecular mass boiling point OH 2-propanol (isopropyl alcohol) 60.1 82.4 °C nitromethane 61.0 101.2 °C
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
nitromethane and 2propanol have similar molecular mass but different boiling points Nitromethane has ...View the full answer

Answered By

Hemstone Ouma
"Hi there! My name is Hemstone Ouma and I am a computer scientist with a strong background in hands-on experience skills such as programming, sofware development and testing to name just a few. I have a degree in computer science from Dedan Kimathi University of Technology and a Masters degree from the University of Nairobi in Business Education. I have spent the past 6 years working in the field, gaining a wide range of skills and knowledge. In my current role as a programmer, I have had the opportunity to work on a variety of projects and have developed a strong understanding of several programming languages such as python, java, C++, C# and Javascript.
In addition to my professional experience, I also have a passion for teaching and helping others to learn. I have experience as a tutor, both in a formal setting and on a one-on-one basis, and have a proven track record of helping students to succeed. I believe that with the right guidance and support, anyone can learn and excel in computer science.
I am excited to bring my skills and experience to a new opportunity and am always looking for ways to make an impact and grow as a professional. I am confident that my hands-on experience as a computer scientist and tutor make me a strong candidate for any role and I am excited to see where my career will take me next.
5.00+
8+ Reviews
22+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
Discussion 1- One key component of descriptive writing is showing the reader something rather than telling the reader. For example, I could tell you that I was cold. Or, I could show you: I shivered,...
-
In order to evaluate lim f(a+h)-f(), it is necessary to evaluate f(a + h). h xa For f(x) = x 3, f(a+h) =
-
Was there sufficient evidence to prove that Reyes violated the AECA and related regulations?
-
Bower Company purchased Lark Corporations net assets on January 3, 20X2, for $625,000 cash. In addition, Bower incurred $5,000 of direct costs in consummating the combination. At the time of...
-
The following TI-84 Plus display presents the results of a test of the null hypothesis H0 : 1 = 0. a. What is the alternate hypothesis? b. What is the value of the test statistic? c. How many degrees...
-
Longhorn Corporation provides low-cost food delivery services to senior citizens. At the end of the year, the company reports the following amounts: In addition, the company had common stock of...
-
4. Find f'(x) for f(x) = 3x+2 4x-5 6. Find the intervals of increasing, decreasing, and constant for f(x) = x-3x-9x + 12. 8. Determine the concavity of the function (concave up and concave down) and...
-
(a) One of the following compounds is an unusual example of a salt that is soluble in hydrocarbon solvents. Which one is it? Explain your choice. (b) Which of the following would be present in...
-
Without consulting tables, arrange the compounds within each of the following sets in order of increasing boiling point, and give your reasoning. (a) 1-hexanol, 2-pentanol, tert-butyl alcohol (b)...
-
The consolidated financial statements of Apple Inc. are given in Appendix A and online in the filings section of www.sec.gov? Requirements 1. 1-Summary of Significant Accounting Policies, under Cash...
-
Egypt was at the center of the storm of the revolutionary activity among Arab nations in the Middle East in 2011-12 and beyond, the Arab Spring Uprisings. Present a factual report and analysis of the...
-
Historically the appreciation rates for different asset classes are not the same. Averaged over the past 40+ years, US stocks grew about 13.8% per year, real estate about 12.5%, foreign stock about...
-
Considering the policies advocated by classical economists, Keynesian economists, and supply-side economist and what you've learned about economic history, which approach do you think is most...
-
Capital costs for a defender are higher than the operating costs, and the annual operating costs are gradually increasing. Capital costs for a defender are less than the operating costs, and the...
-
A knowledge management systemenables organizations to create an environment where knowledge can be captured, processed, stored, and made available for people in the organization that need it. The...
-
The Boston Fire Department receives 911 calls at a mean rate of 1.6 calls per hour (Mass.gov website, November 2012). Suppose the number of calls per hour follows a Poisson probability distribution....
-
The first national bank pays a 4% interest rate compound continuously. The effective annual rate paid by the bank is __________. a. 4.16% b. 4.20% c. 4.08% d. 4.12%
-
Identify the most acidic site in thesecompounds: NH2 CH.COCH,CH,CH3 c) a) b) CH3 e) CH;CH,CH,COH d) CH,CH,CCH,CH3
-
Suggest explanations for the origins of "ibu," "pro," and "fen" in the name ibuprofen. Provide a systematic name for thiscompound OH O,N. NO2 NO2 Picric acid
-
The pK a for the picric acid is 0.42. Explain why it is such a strong acid.
-
Croy Incorporated has the following projected sales for the next five months: Sales in Month April Units 3,400 May 3,825 June 4,640 July August 4,175 3,950 Croy's finished goods inventory policy is...
-
Vulcan Company's contribution format income statement for June is as follows: Vulcan Company Income Statement For the Month Ended June 30 Sales Variable expenses Contribution margin Fixed expenses...
-
Discuss how you would use financial budgets to monitor the performance of responsibility centers.

Study smarter with the SolutionInn App


