(a) One of the following compounds is an unusual example of a salt that is soluble in...
Question:
(a) One of the following compounds is an unusual example of a salt that is soluble in hydrocarbon solvents. Which one is it? Explain your choice.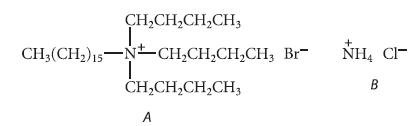
(b) Which of the following would be present in greater amount in a hexane solution of the compound in part (a): separately solvated ions, or ion pairs and higher aggregates? Explain your reasoning.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





