The standard free energy of formation, G f , is the freeenergy change for the formation of
Question:
The standard free energy of formation, ΔG°f, is the freeenergy change for the formation of a substance at 25 °C and 1 atm pressure from its elements in their natural states under the same conditions.
(a) Calculate the equilibrium constant for the interconversion of the following alkenes, given the standard free energy of formation of each. Indicate which compound is favored at equilibrium.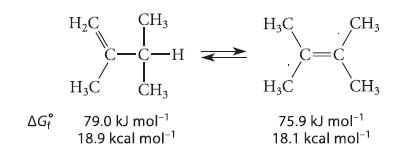
(b) What does the equilibrium constant tell us about the rate at which this interconversion takes place?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





