The two most common forms of glucose, -D-glucopyranose and -D-glucopyranose, can be brought into equilibrium by dissolving
Question:
The two most common forms of glucose, α-D-glucopyranose and β-D-glucopyranose, can be brought into equilibrium by dissolving them in water with a trace of an acid or base catalyst.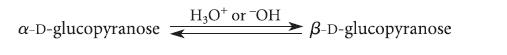
The specific rotation of the equilibrium mixture is 152.7 deg mL g–1 dm–1. The specific rotation of pure a-d-glucopyranose is 1112 degrees mL g–1 dm–1, and that of pure b-d-glucopyranose is 118.7 degrees mL g–1 dm–1.
What is the percentage of each form in the equilibrium mixture?
Transcribed Image Text:
a-D-glucopyranose H₂O* or "OH B-D-glucopyranose
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (2 reviews)
Each compound contributes to the optical activity in prop...View the full answer

Answered By

Aysha Ali
my name is ayesha ali. i have done my matriculation in science topics with a+ . then i got admission in the field of computer science and technology in punjab college, lahore. i have passed my final examination of college with a+ also. after that, i got admission in the biggest university of pakistan which is university of the punjab. i am studying business and information technology in my university. i always stand first in my class. i am very brilliant client. my experts always appreciate my work. my projects are very popular in my university because i always complete my work with extreme devotion. i have a great knowledge about all major science topics. science topics always remain my favorite topics. i am also a home expert. i teach many clients at my home ranging from pre-school level to university level. my clients always show excellent result. i am expert in writing essays, reports, speeches, researches and all type of projects. i also have a vast knowledge about business, marketing, cost accounting and finance. i am also expert in making presentations on powerpoint and microsoft word. if you need any sort of help in any topic, please dont hesitate to consult with me. i will provide you the best work at a very reasonable price. i am quality oriented and i have 5 year experience in the following field.
matriculation in science topics; inter in computer science; bachelors in business and information technology
_embed src=http://www.clocklink.com/clocks/0018-orange.swf?timezone=usa_albany& width=200 height=200 wmode=transparent type=application/x-shockwave-flash_
4.40+
11+ Reviews
14+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The two most common marketing tools used for product advertising are ads on television and ads in a print magazine. Consumers' attitudes toward television and magazine advertising were investigated...
-
The January 2008 edition of Strategic Finance includes an article by Curtis C. Verschoor titled ERC Says Ethics Risk Landscape Still Treacherous. Instructions Read the article and answer the...
-
The two most common isotopes of uranium are 235U and 238U. (a) Compare the number of protons, the number of electrons, and the number of neutrons in atoms of these two isotopes. (b) Using the...
-
DTG agreed to merge with the SPQ. The total equity purchase price of $45,000 is going to be 50% financed issuing DTGs common shares and 50% financed with cash raised by DTG issuing long-term debt....
-
1. The Colorado statute prohibits assisting suicide. Did Dick Bauer violate the statute? 2. If you were on the jury, would you vote to convict?
-
Clinton Corporation spends $800,000 on qualified research activities during the current year. Clintons fixed base percentage is 10%, and the average of its annual gross receipts for the 4 preceding...
-
Police in Albemarle County, Virginia, were on the lookout for a stolen orange and black motorcycle that had eluded them in two previous traffic incidents. Officer David Rhodes drove past the home of...
-
Desiree Clark is a licensed CPA. During the fi rst month of operations of her business, the following events and transactions occurred. May 1 Clark invested $20,000 cash in her business. 2 Hired a...
-
When discussing possible purchase of a fixed immediate annuity, not discussing the full range of available annuitization options and the implications of choosing one over another, particularly with...
-
(a) 2,3,4-Trichloropentane has two meso stereoisomers. Starting with the template below for each, complete line-and-wedge structures for the two meso stereoisomers. (b) Show the symmetry element in...
-
(S)-(1)-Aspartic acid is one of the naturally occurring a-amino acids. Over time in the environment or in aqueous solution, aspartic acid can undergo racemization very slowly (by a process that we...
-
Use nodal analysis to find Vo in the circuit infigure. 4 kn 2 12 V(+ Vo 2 mA 2 +1
-
This project will be known as the meat master online store, and it will be an online platform where customers can place their orders and we will deliver them. This criterion is linked to a Learning...
-
ZYX corporation has ROE of 28% and ROA of 15% what is ZYX's debt to equity ratio?
-
must be based on the document. What are the Different Pricing Strategies? By QuickBooks Canada Team It's no secret that small businesses play a vital role in the Canadian economy. However, revenue...
-
Technology is required to change at a rapid pace due to the dynamics of a global marketplace. Projects require shorter life cycles, and customers are demanding new products and services delivered in...
-
Given that x=2-i is a root of the polynomial x^(3)-x^(2)-7x+15, find all other roots.
-
List several fixed and variable costs associated with owning and operating an automobile. Suppose you are considering whether to drive your car or fly 1000 miles to Florida for spring break. Which...
-
One Way Cellular accountants have assembled the following data for the year ended September 30, 2014: Prepare the operating activities section using the indirect method for One Way Cellulars...
-
Styrene, the simplest alkenylbenzene, is prepared commercially for use in plastics manufacture by catalytic dehydrogenation of ethylbenzene. How might you prepare styrene from benzene using reactions...
-
How would you prepare diphenylmethane, (Ph) 2Ch2, from benzene and an acid chloride?
-
Propose syntheses of the following substances from benzene: (a) m-Chloronitrobenzene (b) m-Chloroethylbenzene (c) 4-Chloro-1-nitro-2-propylbenzene (d) 3-Bromo-2-methylbenzenesulfonic acid
-
Find the accumulated amount A if the principal P is invested at the interest rate of r/year for t years. (Use a 365-day year. Round your answer to the nearest cent.) P= $49,000, r = A $52,797.50 33%,...
-
1) You have a child that is 6 years old, and would like to start saving up for their college education. You would like to be able to pay $24,000 a year for four years of college starting 12 years...
-
Stock Portfolios. A stock portfolio is a collection of stocks that you invest in with the hope of making a profit. Rather than investing everything in a single stock, which has both high risk and...

Study smarter with the SolutionInn App


