When 1-methylcyclohexene undergoes hydration in D 2 O, the product is a mixture of diastereomers; the hydration
Question:
When 1-methylcyclohexene undergoes hydration in D2O, the product is a mixture of diastereomers; the hydration is thus not a stereoselective reaction. (See Fig. P7.57)
(a) Show why the accepted mechanism for this reaction is consistent with these stereochemical results.
(b) Why must D2O (rather than H2O) be used to investigate the stereoselectivity of this addition?
(c) What isotopic substitution could be made in the starting material, 1-methylcyclohexene, that would allow investigation of the stereoselectivity of this addition with H2O?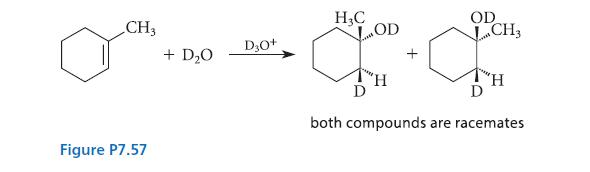
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





