Diphenylmethane exhibits two aromatic rings, which achieve coplanarity in the highest energy conformation. Explain. Diphenylmethane
Question:
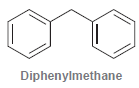
Transcribed Image Text:
Diphenylmethane
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (14 reviews)
Steric hin...View the full answer

Answered By

Issa Shikuku
I have vast experience of four years in academic and content writing with quality understanding of APA, MLA, Harvard and Chicago formats. I am a dedicated tutor willing to hep prepare outlines, drafts or find sources in every way possible. I strive to make sure my clients follow assignment instructions and meet the rubric criteria by undertaking extensive research to develop perfect drafts and outlines. I do this by ensuring that i am always punctual and deliver quality work.
5.00+
6+ Reviews
13+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Biphenyl has the following structure. (a) Is biphenyl a (fused) polynuclear aromatic hydrocarbon? (b) How many pi electrons are there in the two aromatic rings of biphenyl? How does this number...
-
The following compound has two aromatic rings. Identify which ring is expected to be more reactive toward an electrophilic aromatic substitution reaction.
-
Diphenylmethane is significantly more acidic than benzene, and triphenylmethane is more acidic than either. Identify the most acidic proton in each compound, and suggest a reason for the trend in...
-
If one movie ticket costs $13.50, how much will y tickets cost?
-
Research any examples of weaponized industrial network protocols cyber threats and their potential impact to Industrial network protocols. Submit your findings and be sure to include the following:...
-
Show that the Lagrangian density (16.30) is invariant under \(\mathrm{U}(1)\) phase rotations, find the corresponding conserved Noether current, and show that the conserved current is equivalent to...
-
A milk processing unit claims that, of the processed milk converted to powdered milk, \(95 \%\) does not spoil. Find the probabilities that among 15 samples of powdered milk (a) all 15 will not...
-
Knitline Inc. produces high-end sweaters and jackets in a single factory. The following information was provided for the coming year. A sales commission of 5% of sales is paid for each of the two...
-
Three Spirit Think it's time for nights out to change? Cheers to that. Introducing a new kind of nightlife beverage crafted from ancient remedy and modern alchemy. Three Spirit's Social Elixir offers...
-
Joe's Pizza Delivery wants to speed up the ordering process, reduce losses caused by misunderstandings on the phone and attract new customers. Anew web-based pizza ordering system that allows...
-
Would you expect the following compound to be aromatic? Justify your answer. OR N-
-
The following two drawings are resonance structures of one compound: But the following two drawings are not resonance structures: They are, in fact, two different compounds. Explain. Not resonance...
-
Consider an economy where all the agents choose to hold meanvariance efficient portfolios but it is not possible to borrow at the risk free rate \(r_{f}\) (i.e., only investing in the risk free asset...
-
Explain briefly the meaning and importance to auditing of the concept of audit risk.
-
What could a healthcare supervisor or manager do to show support for each core value of the Baldrige Criteria ?
-
List five ways in which CAATs may assist the auditor during an audit engagement.
-
What is marginal product, and what is meant by diminishing marginal product?
-
List seven non-audit services the Sarbanes-Oxley Act of 2002 prohibits auditors from providing to audit clients which are SEC registrants.
-
What is the vacuum wavelength of the 0.186 MeV y-ray emitted by radon-226?
-
The vapor pressure of the liquid NH, is measured at different temperatures. The following vapor pressure data are obtained. Temperature, K P, mmHg 217.1 223.4 234.7 588.1 Calculate the enthalpy of...
-
Consider the second propagation step of peroxide-promoted HBr addition to alkenes (Eq. 5.51b). Explain why hydrogen, and not bromine, is abstracted from HBr by the free radical reactant.
-
Consider the second propagation step of peroxide-promoted HBr addition to alkenes (Eq. 5.51b). Explain why hydrogen, and not bromine, is abstracted from HBr by the free radical reactant.
-
Using the monomer structure in Table 5.4, draw the structure of poly(vinyl chloride) (PVC), the polymer used for the pipes in household plumbing.
-
Discuss the dynamic organization of the cytoskeleton and its pivotal role in cellular motility, intracellular transport, and structural integrity .
-
Would you support the idea of a government issued Digital currency? Why ? and why not?
-
To protect her savings against further inflation and to help her prepare for a healthy financial future, Hanna Lind deposits $9,100 in an investment account earning 6% interest compounded quarterly....

Study smarter with the SolutionInn App


